
Feb. 17, 2022
Feature Story
Cyanide and Life on Early Earth
Researchers supported by the NASA Astrobiology Program have found that cyanide could have helped to enable important metabolic reactions for early life on Earth. Cyanide is well-known today as a poison for organisms like humans, but billions of years ago it may have played a different role for life on our planet.
When life first arose on Earth, the conditions on our planet were dramatically different than they are today. Scientists have long been studying the potential chemistry of the early Earth and how the available chemicals could have been utilized to form the first biological reactions of life. Cyanide is one such chemical that could have been present at the time. Some theories suggest that hydrogen cyanide was delivered to the Earth by meteorites. In the famous Miller-Urey experiment, hydrogen cyanide was an intermediate in the chemical reactions that produced amino acids when an experimental prebiotic mixture was struck with a spark of electricity to simulate lightning.
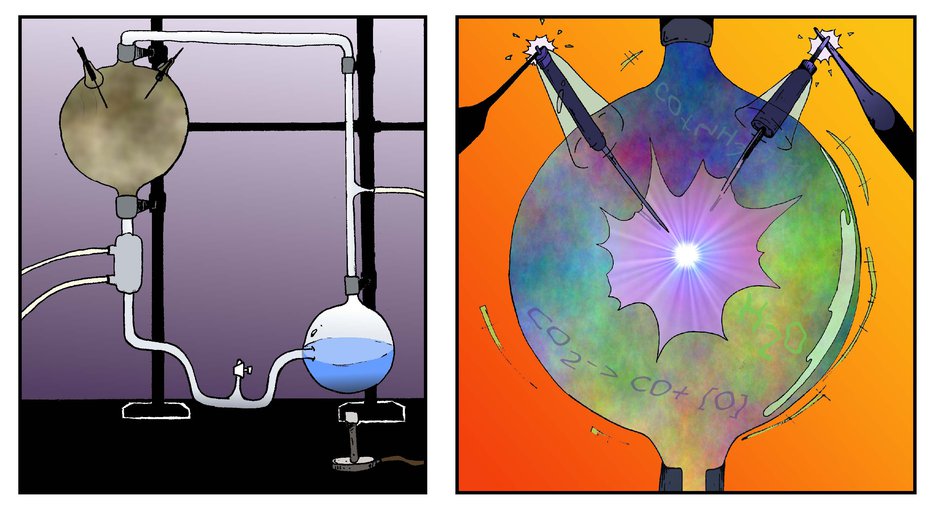
In 1952, Miller-Urey experiment started with water, ammonia, hydrogen, and methane; which, at the time, were considered to be potential components of the Earth’s early atmosphere. After being shocked with electricity, this mixture produced glycine, α-alanine, and β-alanine. Aspartic acid and α-aminobutyric acid (AABA) were also likely produced, although their identification was less certain with the detection techniques used. Hydrogen cyanide was an important intermediate in the chemical reactions that led from the starting material to the amino acid products. The experiment features in Issue 1 of the Astrobiology Graphic Histories, available at: https://astrobiology.nasa.gov/resources/graphic-histories/Image credit: NASA Astrobiology / Aaron Gronstal.
In the new study, researchers found that cyanide can be used in reactions that form carbon-based compounds from carbon dioxide (CO2). Current theories suggest that CO2 could have been a considerable component of the early atmosphere. Importantly, cyanide helps these reactions occur without the use of complex proteins that are used by life today. The team believes that the findings could help astrobiologists better understand the evolution of life on Earth, as well as the potential for life on other worlds.
The study, “Cyanide as a primordial reductant enables a protometabolic reductive glyoxylate pathway,” was published in the journal Nature Chemistry.
Click here for a press release concerning this research from Scripps Research.
The research was supported by NASA Astrobiology through the Exobiology Program and the NSF/NASA Center for Chemical Evolution.