2014 Annual Science Report
 University of Illinois at Urbana-Champaign
Reporting | SEP 2013 – DEC 2014
University of Illinois at Urbana-Champaign
Reporting | SEP 2013 – DEC 2014
Project 8 Culturing Microbial Communities in Controlled Stress Micro-Environments
Project Summary
This project explores the adaptation and evolution of organisms under controlled environmental conditions, and compares the behavior across two Domains of Life in order to identify and quantify universal aspects of evolutionary response.
Project Progress
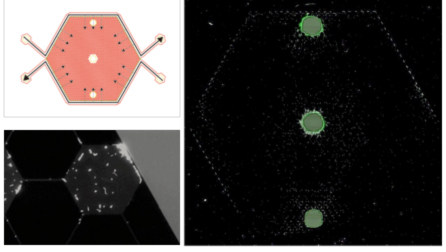
Our team has designed and built the GeoBioCell —- a microfluidic gradient chamber created by etching a 1200-well array and boundary channels into a silicon wafer using photolithography methods. Archaea Methanosarcina acetivorans and Bacteria Escherichia coli were chosen as the model organisms to evaluate, and constraint conditions were created by establishing a concentration gradient within the GeoBioCell of an inhibitory antibiotic, i.e., puromycin for M. acetivorans and ciprofloxacin for E. coli. Cell growth was evaluated in batch systems for both M. acetivorans and E. coli in the presence of the antibiotic to determine the minimum inhibitory concentrations (MICs). The GeoBioCell design and results of E. coli growth in the presence of a ciprofloxacin are illustrated in Figure 1. Substrates (LB media, dissolved O2) were continuously delivered to the boundary channels of the GeoBioCell, while ciprofloxacin was continuously delivered to only the bottom boundary channel to establish a concentration gradient. E. coli cells were inoculated into the center port; they are labeled with a green fluorescence protein (GFP) so growth was tracked using fluorescent microscopy. Cells began to visibly grow and spread within hours, and after 30 hours (Fig. 1) E. coli have explored the entire 1200-well array and are primarily growing in wells along the upper boundary channel where ciprofloxacin concentration is lowest. There is also some E. coli in wells along the bottom left channel, due to dilution of ciprofloxacin as it flows from right to left through the boundary channel. Under higher magnification (Fig. 1), individual cells are observable in a single well. We note that along the bottom boundary toward the inlet individual cells were also visible, but they were not fluorescing (implying they are not replicating). No adaptation to ciprofloxacin was observed in this experiment, even after two weeks. We hypothesis that this is due to limited O2 diffusion from the boundary channels, and have redesigned the GeoBioCell with a gas permeable coverslip to allow O2 diffusion directly into all 1200-wells in our next experiment.
Publications
-
Boyd, V., Yoon, H., Zhang, C., Oostrom, M., Hess, N., Fouke, B., … Werth, C. J. (2014). Influence of Mg2+ on CaCO3 precipitation during subsurface reactive transport in a homogeneous silicon-etched pore network. Geochimica et Cosmochimica Acta, 135, 321–335. doi:10.1016/j.gca.2014.03.018
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Bruce Fouke
Project Investigator
Isaac Cann
Co-Investigator
Yiran Dong
Co-Investigator
Rod Mackie
Co-Investigator
Charles Werth
Co-Investigator
Reinaldo Alcalde
Collaborator
Robert Sanford
Collaborator
Lang Zhou
Collaborator
-
RELATED OBJECTIVES:
Objective 6.1
Effects of environmental changes on microbial ecosystems
Objective 6.2
Adaptation and evolution of life beyond Earth