2014 Annual Science Report
 University of Illinois at Urbana-Champaign
Reporting | SEP 2013 – DEC 2014
University of Illinois at Urbana-Champaign
Reporting | SEP 2013 – DEC 2014
Project 4: Rapid Evolution in Stressed Populations: Theory
Project Summary
Evolution is typically thought of as occurring over millions of years. But recently it has become clear that we have grossly over-estimated this time scale. Perhaps the most famous example of this is the rapid evolution of resistance of bacteria, worldwide, to modern antibiotics. Similarly, early life has an evolution time scale problem: given the age of the Earth and the known age of the Last Universal Common Ancestor, life must have arisen and evolved the majority of the complexity of the modern cell in less than a billion years. This project is a theoretical attempt to understand how a fluctuating environment can accelerate evolution rate, and lead to evolution on ecosystem time scales. Eventually, this work will join up with the experimental work being done by our team, using the GeoBioCell, in Project 8.
Project Progress
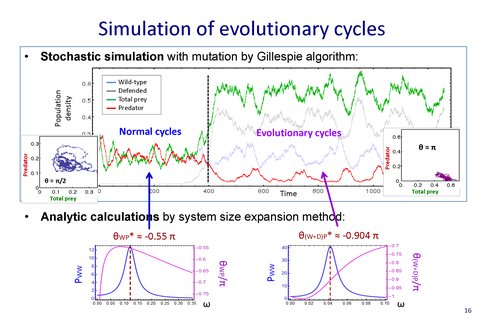
A key problem in astrobiology is what governs the rate of evolution, and how is this rate influenced by the environment? This question is being explored experimentally in Theme 4 from our original proposal. In tandem with the ongoing experiments on bacterial and archaeal stress response in microfluidic engineered environments, we have performed a detailed stochastic individual level calculation of the way in which environmental fluctuations can drive ecological processes and vice versa. In particular, genetic variation in a population can sometimes arise so fast as to modify ecosystem dynamics. Such phenomena have been observed in natural predator-prey systems and characterized in the laboratory as showing unusual phase relationships in population dynamics, including a pi phase shift between predator and prey (evolutionary cycles) and even undetectable prey oscillations compared to those of the predator (cryptic cycles). We were able to develop a generic individual-level stochastic model of interacting populations that includes a subpopulation of low nutritional value to the predator. Using a master equation formalism and by mapping to a coherent state path integral solved by a system-size expansion, we showed that evolutionary and cryptic quasicycles can emerge generically from the combination of intrinsic demographic fluctuations and clonal mutations alone, without additional biological mechanisms. The main result from this work is that evolutionary timescales can be accelerated significantly and indeed may occur on the same scale as environmental variability, not much longer as is conventionally assumed. The specific setting for this work was an ecosystem comprising predators and prey, but where it was possible for a mutant prey strain to arise, one which is both less able to compete with the wild-type for nutrient and is also less nutritious to the predator. However, the ideas are general and expected to apply in any system where there are large environmental variations or spatial gradients. Our work showed that these phenomena are rather universal, and do not require rather detailed and specific forms of functional response, as has been proposed up to now. These findings provide a framework that can explain the rapid evolutionary dynamics that has been reported in laboratory experiments, and which are the subject of Theme 4.
Publications
-
Shih, H-Y., & Goldenfeld, N. (2014). Path-integral calculation for the emergence of rapid evolution from demographic stochasticity. Phys. Rev. E, 90(5), None. doi:10.1103/physreve.90.050702
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Charles Werth
Project Investigator
Bruce Fouke
Co-Investigator
Nigel Goldenfeld
Co-Investigator
Hong-Yan Shih
Collaborator
-
RELATED OBJECTIVES:
Objective 4.1
Earth's early biosphere.
Objective 4.2
Production of complex life.
Objective 5.1
Environment-dependent, molecular evolution in microorganisms
Objective 5.2
Co-evolution of microbial communities
Objective 5.3
Biochemical adaptation to extreme environments
Objective 6.1
Effects of environmental changes on microbial ecosystems