2011 Annual Science Report
 Montana State University
Reporting | SEP 2010 – AUG 2011
Montana State University
Reporting | SEP 2010 – AUG 2011
Reactivity of Pyrite Surfaces With Thiomolybdate as Sorbate
Project Summary
Sorption of a sulfur-molybdenum compound onto pyrite surfaces leads to an enhance capability of pyrite to facilitate the conversion of dinitrogen to ammonia, a key reagent in the formation of amino acids on the prebiotic Earth. The structure and chemical environment of the molybdenum-sulfur surface compound is thought to be similar to molybdenum-sulfur compounds embedded in enzymes, where these compounds facilitate the conversion of dinitrogen to ammonia.
Project Progress
Prior studies have generally concluded that the dinitrogen activation on iron sulfide surfaces—including the surface of pyrite-is kinetically hindered and likely did not contribute significantly to nitrogen fixation in the Hadean. However, this notion is based on experiments with pristine iron sulfide surfaces. Experiments within this funding period showed that adsorption of tetrathiomolybdate (MoS42-) on the pyrite surface results in a surface that can facilitate the conversion of nitrate to ammonium at significantly lower temperatures than the pristine pyrite surface (70°C vs 120°C).
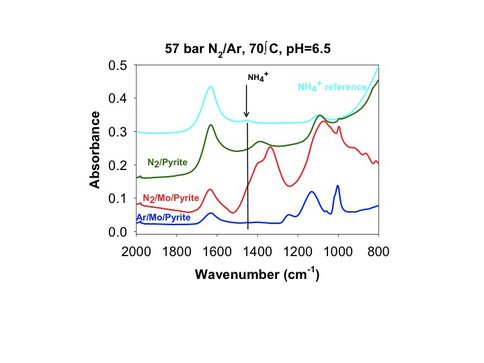
Inspired by these results, a set of in situ Fourier Transform infrared spectroscopy (ATR-FTIR) experiments was conducted to evaluate if the pyrite surface with tetrathiomolybdate can facilitate the reduction of dinitrogen to ammonia. The results of the ATR-FTIR experiments show that dinitrogen can be activated to form ammonia on the Mo-modified pyrite surface in the aqueous phase at relatively low temperature (70°C). Small-scale batch experiments are underway to corroborate the results of these ATR-FTIR experiments.
The ATR-FTIR results suggest that nitrogen fixation on early Earth may have been facilitated by modified pyrite surfaces. This finding gives a possible direct route to ammonia from dinitrogen in contrast to the conversion of nitrogen oxides to ammonium on early Earth by aqueous Fe(II) or FeS, which rests on the premise that electrical discharges could have produced such species from an atmosphere that likely contained both nitrogen and carbon dioxide.
The activation of the pyrite surface is likely due to the presence of Mo-Fe-S cubane structures. The presence of such structures is consistent with prior research that has identified such complexes with X-ray absorption spectroscopy (XAS). The Mo-Fe-S cubane is a structural motif that may have been recruited by some of the first forms of cellular life and evolved into the metal center of nitrogenase.
Preliminary attenuated total reflectance infrared data that shows that vibrational bands associated with ammonium develop on Mo-modified pyrite in the presence of dissolved N2. The presence of ammonium has been verified by an analysis of the aqueous phase with ion chromatography.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Martin Schoonen
Co-Investigator
Alexander Smirnov
Co-Investigator
Robert Szilagyi
Co-Investigator
Michael Vance
Postdoc
Alex Gordon
Doctoral Student
Soujanya Singireddy
Doctoral Student
-
RELATED OBJECTIVES: