2011 Annual Science Report
 Montana State University
Reporting | SEP 2010 – AUG 2011
Montana State University
Reporting | SEP 2010 – AUG 2011
Paradigms for Complex Iron-Sulfur Cluster Assembly and the Origin and Evolution of Iron-Sulfur Enzymes
Project Summary
We have presented seminal results in the past year that define paradigms for iron-sulfur cluster assembly in biology that are shared between several important enzymes systems. This work has allowed for the formulation of new models for the origin and evolution of iron sulfur enzymes. An evolutionary origin that involves a mineral beginning and the stepwise refinement of catalytic function in response to selective pressure.
Project Progress
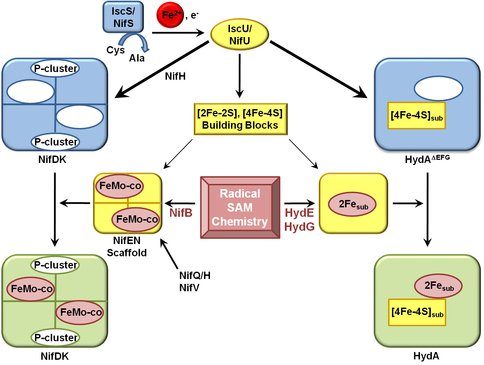
We have made seminal progress in our understanding of complex biological iron-sulfur cluster assembly during this past year. The results of our biochemical work coupled with a parallel phylogenetic analysis of complex iron-sulfur enzymes and their closely relatives has provided for significant advances in our ability to rationalize how at least a subset of complex iron-sulfur enzymes evolved. The main breakthrough from the past year was the structure of an [FeFe]-hydrogenase which was captured prior to insertion of its complex iron-sulfur cluster. In place of the cluster the structure revealed an open cavity and perhaps more importantly the structure of the evolutionary ancestor. We have made strong connections between the biosynthetic pathways for maturation of [FeFe]-hydrogenases and nitrogenases and have brought to light that both systems involved similar simple cluster precursors, radical chemistry, and nucleotides (Figure 1). For nitrogenase it has been known for sometime as well that a form of nitrogenase without its complex FeMo-cofactor can exist in a stable state and recently it was also revealed that its closest evolutionary relative involved in chlorophyll biosynthesis (protocholorophylide reductase) is similar to this nitrogenase intermediate.
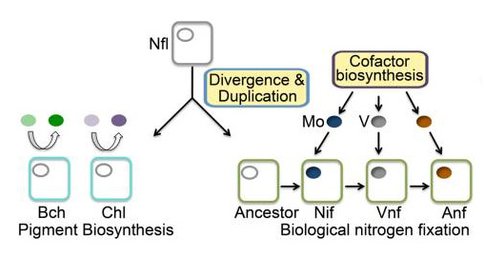
The culmination of these results have lead to the proposal of a new unifying theory for the evolutionary origin of nitrogenase and [FeFe]-hydrogenases. We believe now that both complex iron-sulfur enzymes were evolved from an ancestral protein with an open cavity. The cavity was capable of binding metals and metal clusters that could lead to a selectable catalytic activity; for example the ability to catalyze dinitrogen reduction, hydrogen oxidation, or proton reduction. In biology the generation of cluster or modified by an enzyme activity altered by mutation could be selected for. Subsequent modifications could be added in a stepwise fashion in a similar manner. The scheme is to our knowledge the first theory for the evolutionary origin of complex iron-sulfur enzymes marries the current knowledge of iron-sulfur enzyme biochemistry with the details of protein evolution. For the nitrogenase we believe that the ancestor of nitrogenase and protochlorophylide is a protein thought to be involved in nickel cofactor biosynthesis in methanogenesis and thus an open cavity capable of binding and nickel porphyrin (Figure 2).
Pathways for maturation of the Mo-nitrogenase and the [FeFe]-hydrogenase The biogenesis of both the FeMo-cofactor and the H-cluster begins with the utilization of the housekeeping iron-sulfur cluster assembly machinery to generate simple iron sulfur cluster building blocks that are subsequently modified. In both cases, these clusters are transferred to the structural proteins which already have a complement of iron-sulfur clusters (P clusters for nitrogenase and [4Fe-4S] subcluster of the H cluster for [FeFe]-hydrogenase. . Final maturation and generation of an active nitrogen or [FeFe]-hydrogenase occurs by the insertion of the modified cluster (FeMo-cofactor or H cluster 2Fe subcluster) into a cofactorless structural protein through cationically charged channels.
Model depicting the divergence of nitrogenase (NifD) and protochlorophyllide reductase (ChlN/BchN) from a NflD ancestor. The stepwise evolution of cofactor biosynthesis leading to the acquisition of metal specificity in the covalently bound active site metallocluster, where Mo acquisition and Mo-nitrogenase predates V acquisition and V-nitrogenase, and V acquisition predates Fe-only nitrogenase. ChlN/BchN bind substrates in their active site cavities non-covalently and release these substrates following reduction (Muraki et al., 2010). Abbreviations: Mo, molybdenum; V, vanadium; Nif, Mo-dependent nitrogenase; Vnf, V-dependent nitrogenase; Anf, Fe-only nitrogenase; Bch, BchN protein involved in bacteriochlorophyll biosynthesis; Chl, ChlN protein involved in chlorophyll biosynthesis.
Publications
-
Boyd, E. S., Anbar, A. D., Miller, S., Hamilton, T. L., Lavin, M., & Peters, J. W. (2011). A late methanogen origin for molybdenum-dependent nitrogenase. Geobiology, 9(3), 221–232. doi:10.1111/j.1472-4669.2011.00278.x
-
Boyd, E. S., Hamilton, T. L., & Peters, J. W. (2011). An Alternative Path for the Evolution of Biological Nitrogen Fixation. Frontiers in Microbiology, 2. doi:10.3389/fmicb.2011.00205
-
Mulder, D. W., Boyd, E. S., Sarma, R., Lange, R. K., Endrizzi, J. A., Broderick, J. B., & Peters, J. W. (2010). Stepwise [FeFe]-hydrogenase H-cluster assembly revealed in the structure of HydAΔEFG. Nature, 465(7295), 248–251. doi:10.1038/nature08993
-
Mulder, D. W., Shepard, E. M., Meuser, J. E., Joshi, N., King, P. W., Posewitz, M. C., … Peters, J. W. (2011). Insights into [FeFe]-Hydrogenase Structure, Mechanism, and Maturation. Structure, 19(8), 1038–1052. doi:10.1016/j.str.2011.06.008
-
Shepard, E. M., Boyd, E. S., Broderick, J. B., & Peters, J. W. (2011). Biosynthesis of complex iron–sulfur enzymes. Current Opinion in Chemical Biology, 15(2), 319–327. doi:10.1016/j.cbpa.2011.02.012
-
Soboh, B., Boyd, E. S., Zhao, D., Peters, J. W., & Rubio, L. M. (2010). Substrate specificity and evolutionary implications of a NifDK enzyme carrying NifB-co at its active site. FEBS Letters, 584(8), 1487–1492. doi:10.1016/j.febslet.2010.02.064
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Eric Boyd
Co-Investigator
Joan Broderick
Co-Investigator
Eric Shepard
Postdoc
David Mulder
Doctoral Student
Alta Howells
Undergraduate Student
Rachel Lange
Undergraduate Student
-
RELATED OBJECTIVES:
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules
Objective 3.3
Origins of energy transduction