2011 Annual Science Report
 Montana State University
Reporting | SEP 2010 – AUG 2011
Montana State University
Reporting | SEP 2010 – AUG 2011
Nitrate and Nitrate Conversion to Ammonia on Iron-Sulfur Minerals
Project Summary
Conversion of nitrate and nitrite may have contributed to the formation of ammonia—a key reagent in the formation of amino acids—on the prebiotic Earth. Results suggest that the presence of iron mono sulfide facilitates the conversion of nitrate and nitrite. Nitrite conversion is, however, much faster than the conversion of nitrate.
Project Progress
Nitrate and nitrite, generated by dissolution of NOx during lightning strikes, may have been an important source of reactive nitrogen in the Hadean. We evaluated the production of ammonia from nitrate and nitrite in aqueous solutions containing freshly precipitated FeS. Our studies suggest that a novel pathway may be operative where ammonia formation is driven by a disproportionation reaction of nitrite (NO2-) to nitrate (NO3-) and nitric oxide (NO), with the latter species becoming further reduced in concomitant steps to ammonia.
A systematic experimental effort in which we evaluated the production of ammonia from nitrate and nitrite in aqueous solutions containing freshly precipitated FeS was completed to determine the effect of temperature on the conversion kinetics. Surface complexes were indentified using attenuated total reflection Fourier Transform infrared spectrophotometer (ATR-FTIR), which eliminated the intense water absorbances found in traditional FTIR measurements and allowed detection of signals for species adsorbed on the surface in low concentrations.
Complementary small-scale batch experiments yielded kinetic measurements. Included in these experiments were a series of control experiments to determine the stability of the nitrite ion and nitrate in water as well as the release of ammonia from the FeS. Freshly precipitated FeS was synthesized by rapidly mixing a ferrous sulfate solution and a sodium sulfide solution. The FeS precipitate has an average crystallite size of several nm. All kinetic data were corrected for the rate of ammonia release in the control experiments.
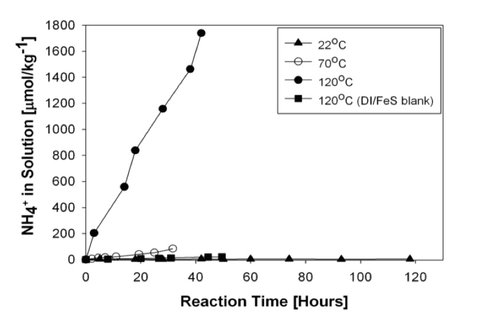
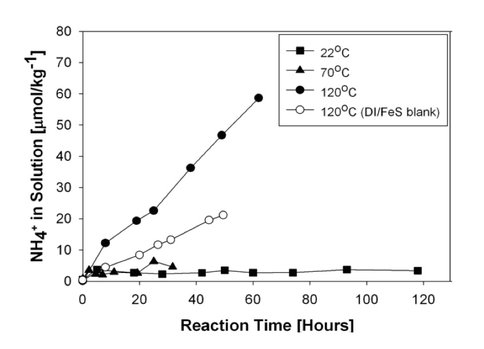
The kinetic results show that a significant amount of ammonia forms in the nitrite/FeS system at 120°C. As shown in the figure below, within 40 hours close to 1.8 mmoles of ammonia are produced per kg of solution. This system remains reactive even at lower temperatures (see top panel figure). The nitrate/FeS system is less reactive, but does show ammonia formation at 120°C (see bottom panel of figure).
The results of this study provide a detailed insight into the mechanism of ammonia formation from nitrite and nitrate reduction. Furthermore, the kinetics suggest that some nitrite or nitrate that entered into an ocean with FeS in the water column would of been converted to ammonia, in particular if the ocean was warmer than it is now.
Publications
-
Gordon, A. D., Smirnov, A., Shumlas, S. L., Singireddy, S., DeCesare, M., Schoonen, M. A. A., & Strongin, D. R. (2013). Reduction of Nitrite and Nitrate on Nano-dimensioned FeS. Orig Life Evol Biosph, 43(4-5), 305–322. doi:10.1007/s11084-013-9343-4
-
Singireddy, S., Gordon, A. D., Smirnov, A., Vance, M. A., Schoonen, M. A. A., Szilagyi, R. K., & Strongin, D. R. (2012). Reduction of Nitrite and Nitrate to Ammonium on Pyrite. Orig Life Evol Biosph, 42(4), 275–294. doi:10.1007/s11084-012-9271-8
- Gordon, A.D., Smirnov, A., Shumlas, S., Schoonen, M.A.A. & Strongin, D.R. (2011). Effect of Molybdenum on the reduction of nitrate and nitrite on pyrite. In preparation.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Alex Gordon
Doctoral Student
Alexander Smirnov
Doctoral Student
Soujanya Singireddy
Unspecified Role
-
RELATED OBJECTIVES:
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules
Objective 3.3
Origins of energy transduction
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems