2011 Annual Science Report
 Carnegie Institution of Washington
Reporting | SEP 2010 – AUG 2011
Carnegie Institution of Washington
Reporting | SEP 2010 – AUG 2011
Project 4: Geochemical Steps Leading to the Origins of Life
Project Summary
This project involves research designed to aid understanding the geochemical roots of life focusing in particular on the role of mineral surfaces play in catalyzing organic reactions that may have biochemical utility.
Project Progress
4.1 Geochemical Roots of Life
During the past funding period, NAI post-Doc Ohara and CoI Cody continued their studies of mineral catalyzed peptide formation. There have been a number of previous studies that reveal pathways to peptide synthesis at moderately dilute amino acid concentrations using either excess carbon monoxide or excess carbonyl sulfide (COS). Such high concentrations of either molecule appear unlikely and Ohara and Cody have found that peptide formation is significantly enhanced in the presence of pyrite (FeS2), chalcopyrite (CuFeS2) or sphalerite (ZnS) in the absence of COS or CO.
Ohara and Cody have continued studying reactions of 200 microL of 100 mM glycine solution with 10.0-200.0 mg of fine grained pyrite (Haunzala, Peru), chalcopyrite (Mexico) and sphalerite (New York, USA). Reactions are performed under vapor pressure and at temperatures of 60-100°C for multiple days. Control experiments (without mineral) were also run under identical conditions. Quantitative analysis of glycine and peptides was performed with electrospray ionization-liquid chromatography-mass spectrometry (ESI-LC-MS) detection. The fact that peptide formation is surface-catalyzed was suggested by the fact that the yield strongly correlated with mineral surface area. Interestingly, the yield of di-peptide (Gly2) with ZnS or CuFeS2 was much higher than with FeS2 under the same surface-are conditions. The ratio of the yield of Gly2 with ZnS to that without mineral increased with decreasing temperature, and reached 80 at 60°C. Peptization reaction with ZnS proceeded up to tri-peptide (Gly3), which was not detected in the control at any temperature. The apparent activation energy for mineral catalyzed peptide formation is ~ ½ that of the mineral absent control indicating that the amino acid-mineral surface interaction does not just increase the effective molarity (i.e., local concentration), but rather some specific amino acid mineral surface interaction occurs that lowers the activation energy barrier to amino acid condensation. During the past year Ohara and Cody have been using Attenuated Total Reflectance (ATR) Fourier Transform InfraRed (FTIR) spectroscopy to investigate, directly, amino acid mineral surface interactions and have initiated quantum mechanical calculations in parallel to aid interpretation of the origins of surface perturbed vibrational modes. Preliminary results of this work were presented at a special Astrobiology session held at the 2010 PacifiChem meeting in December. A manuscript detailing this work is in preparation for submission.
Overall, these results suggest that high-temperature (> 100°C) conditions are not required nor optimal for peptide formation in the presence of sulfide mineral. This suggests that cooler environments in close proximity to metal sulfide precipitating hydrothermal systems may have been advantageous to the emergence of life on the early Earth.
4.2 Prebiotic Molecular Selection and Organization
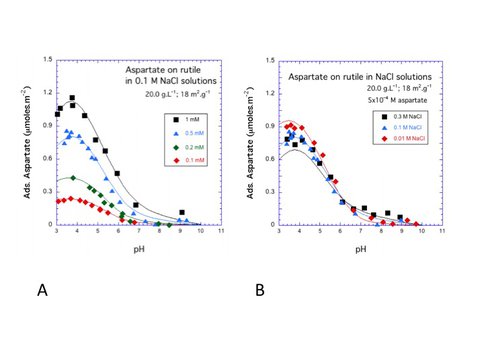
During the last funding period we have continued to focus on organic-mineral surface interactions. A key objective of our program is to explore mechanisms by which prebiotic molecules may have been selected and concentrated in plausible geochemical environments, especially on mineral surfaces. We obtained fundamental data on speciation and coordination chemistry of amino acids on mineral surfaces by studies of aspartic acid and dihydroxyphenylalanine (DOPA) on rutile as functions of pH, ionic strength, and ligand-to-solid ratio using potentiometric titration and batch adsorption experiments. We integrated data with the extended triple-layer model (ETLM), a surface complexation model that enables insights into the number of inner-sphere attachment points for the amino acids at the surface. Representative adsorption data for aspartate are shown in Figure 2.
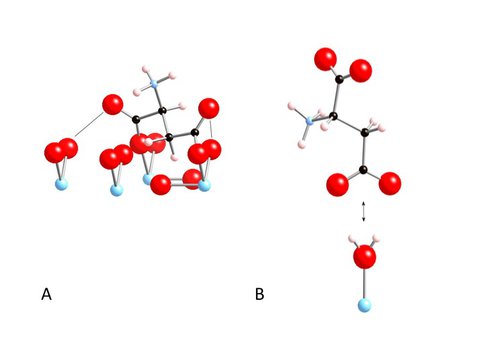
These experimental data provide strong constraints on the stoichiometry of the adsorption reactions. The surface complexation model required two surface species as shown in Figure 3. Calculated proportions of the two surface aspartate species vary strongly with pH and surface loading.
Our aspartate surface species and previously published glutamate species are consistent with ATR-FTIR spectroscopic results for both amino acids and with quantum chemical studies glutamate adsorption on rutile. It is interesting that the quantum chemical calculations support the same type of surface species originally suggested from the ETLM analysis of the adsorption and surface titration data.
Our study of DOPA adsorption to rutile illustrates the importance of the binding of catecholic -OH groups to an oxide surface. Dihydroxyphenylalanine (DOPA) and similar molecules are of considerable interest in studies of bioadhesion to minerals, solar cells involving titanium dioxide and biomedical imaging. However, the extent and mechanisms of DOPA adsorption on oxides in salt solutions are unknown. We reported measurements of DOPA adsorption on well-characterized rutile (α-TiO2) particles over a range of pH, ionic strength and surface coverage as well as a surface complexation model analysis establishing the stoichiometry, model surface speciation and thermodynamic equilibrium constants, which permits predictions in more complex systems. DOPA forms two surface species on rutile, the proportions of which vary strongly with pH but weakly with ionic strength and surface loading. At pH < 4.5 a species involving four attachment points (“lying down”) is important, whereas at pH > 4.5 a species involving only two attachment points via the phenolic oxygens (“standing up”) predominates. Based on evidence of strong attachment of DOPA to titanium dioxide from single molecule AFM and studies of catechol adsorption, one or more of the DOPA attachments for each species is inner-sphere, the others are likely to be H-bonds.
Overall, important conclusions of this multi-technique study include:
(1) Our results for glutamate attachment to rutile are consistent with adsorption and proton surface titration experiments, ATR-FTIR spectroscopic data, and surface complexation and quantum chemical studies. This study provides the most thoroughly documented amino acid/mineral interaction in the literature. It serves as a model system for understanding amino acid interactions with mineral surfaces in more complex systems.
(2) Amino acids containing two carboxylate or two adjacent hydroxyl groups form inner sphere (chemisorbed) complexes with Ti-O bonds which points to a possible role of mineral surfaces in selecting and protecting key prebiotic molecules.
(3) Multiple competing amino acid surface species occur (Fig. 2) and the relative proportions of these vary significantly with pH and solute concentration.
(4) Amino acids lying down on the surface attcahed at four points may be relevant to chiral selectivity. Amino acids standing up on the surface attached at two points by the side chain carboxylate may be important for peptide bond formation.
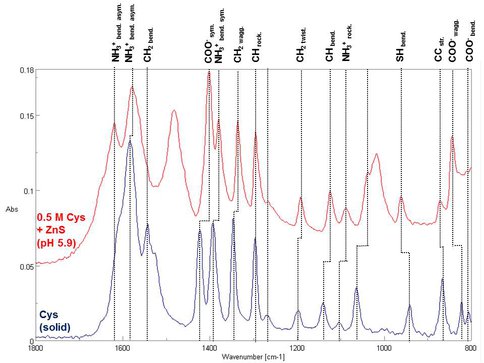
Fig 4.1: Atenuated Total Reflectance (ATR) Fourier Transform InfraRed (FTIR) spectra of the amino acid cysteine in solution (top-red) and on the surface of the mineral sphalerite (ZnS). The shifts in vibrational band frequencies and intensities reveal that the geometry of cysteine on the ZnS surface differs significantly than that in aqueous solution indicating the role of specific interactions of the amino acid functional groups and active functional groups on the ZnS surface. The observation of such interactions will aid in our understanding of how ZnS catalyzes the polymerization of amino acids.
Fig 2. Symbols represent batch adsorption experiments as a function of surface loading (A) and ionic strength (B) for aspartate on rutile as a function of pH and aspartate concentration. Curves represent surface complexation calculations based on the model of Sverjensky & Fukushi (2006).
Fig 3. Modes of aspartate attachment to rutile include bridging-bidentate species with binding to both carboxyl groups (A); an outer-sphere species, which binds only to the γ-carboxyl group (B). Atoms of Ti, O, C, and H are colored blue, red, black, and pink, respectively.
Publications
Papers:
2010: Marshall-Bowman, K., Ohara, S., Sverjensky, D. A., Hazen, R. M., and Cleaves, H. J., Catalytic peptide hydrolysis by mineral surface: Implications for prebiotic chemistry. Geochim. Cosmochim. Acta 74, 5852-5861.
2011: Parikh, S. J., Kubicki, J. D., Jonsson C. M., Jonsson C. L., Hazen, R. M., Sverjensky D. A. and Sparks, D. L., Evaluating glutamate and aspartate binding mechanisms to rutile (α-TiO2) via ATR-FTIR spectroscopy and quantum chemical calculations. Langmuir, v. 27, pp. 1778-1787.
2011: Bahri, S., Jonsson, C. M., Jonsson C. L., Azzolini, D., Sverjensky, D. A. and Hazen R. M., Adsorption and surface complexation study of L-DOPA on rutile (α-TiO2) in NaCl solutions. Environmental Science & Technology, v. 45, pp. 3959-3966.
Abstracts:
2010: Lee, N., Jonsson, C. M., Jonsson, C. L., Klochko, K., Cleaves, H. J., Sverjensky, D. A. and Hazen, R. M. Interactions of L-lysine with rutile in NaCl solutions. 239th Amer. Chem. Soc. National Meeting (San Francisco, CA).
2010: Klochko, K., Jonsson, C. M., Jonsson, C. L., Lee, N., Cleaves, H. J., Sverjensky, D. A. and Hazen, R. M. L-arginine adsorption and surface complexation on rutile in NaCl solutions. 239th Amer. Chem. Soc. National Meeting (San Francisco, CA).
2010: Klochko, K., Jonsson, C. M., Jonsson, C. L., Lee, N., Cleaves, H. J., Sverjensky, D. A. and Hazen, R. M. Role of Environmental Conditions on the Interaction of L-Arginine with Oxide Mineral Surfaces. Astrobiology Science Conference (League City, TX).
2010: Lee N., Jonsson C. M., Jonsson C. L., Ohara S., Cody G., Klochko K., Cleaves J. H., Sverjensky D. A. & Hazen R., L-Lysine Adsorption on Oxide Surfaces as a Function of Environmental Conditions. Astrobiology Science Conference (League City, TX).
2010: Sverjensky D. A., Jonsson C. M., Jonsson C. L., Estrada C., Lee N., Klochko K., Cleaves H. J., Hazen R. M., Parikh S., Kubicki J. D. & Sparks D. L., Attachment of Acidic Amino Acids to Mineral Surfaces: Implications for Prebiotic Chemistry. Goldschmidt Geochemistry Conference (Knoxville, TE).
2010: Lee N., Jonsson C. M., Jonsson C. L., Ohara S., Cody G., Klochko K., Cleaves J. H., Sverjensky D. A. & Hazen R., Adsorption of Amino Acids on Oxide Surfaces as a Function of Environmental Conditions. Goldschmidt Geochemistry Conference (Knoxville, TE).
Fall Meeting Invited talk.
2011: Lee, N., Foustoukos, D. I., Sverjensky D. A., Cleaves, H. J. II, Klochko, K. & Hazen R. M., The stability of amino acids under redox-constrained hydrothermal conditions. Goldschmidt Geochemistry Conference (Prague, CZ).
2011: Villegas-Jimenez, A., Hazen R. M. & Sverjensky D. A. Novel insights into the ion sorption properties of calcite in aqueous solutions using cavity ring-down spectroscopy. Goldschmidt Geochemistry Conference (Prague, CZ).
2011: Livi, N., Schaffer, B., Azzolini, D., Hardcastle, T., Seabourne, C. R., Scott, A. J., Sverjensky D. A., Hazen R. M. & Brydsen, R. Surface structures on rutile guide organic molecule attachment. Goldschmidt Geochemistry Conference (Prague, CZ).
2011: Klochko, K., Sverjensky D. A., Hazen R. M. & Cleaves, H. J. II. The role of mineral surface chemistry in the prebiotic selection of pentose sugars. Goldschmidt Geochemistry Conference (Prague, CZ).
2011: Sverjensky D. A., Hazen R. M., Azzolini, D., Lee, N. & Klochko, K. Surface complexation evidence that amino acids prefer special sites on oxide surfaces. Goldschmidt Geochemistry Conference (Prague, CZ).
Publications
-
Bahri, S., Jonsson, C. M., Jonsson, C. L., Azzolini, D., Sverjensky, D. A., & Hazen, R. M. (2011). Adsorption and Surface Complexation Study of L-DOPA on Rutile (α-TiO 2 ) in NaCl Solutions. Environ. Sci. Technol., 45(9), 3959–3966. doi:10.1021/es1042832
-
Grew, E. S., Bada, J. L., & Hazen, R. M. (2011). Borate Minerals and Origin of the RNA World. Orig Life Evol Biosph, 41(4), 307–316. doi:10.1007/s11084-010-9233-y
-
Marshall-Bowman, K., Ohara, S., Sverjensky, D. A., Hazen, R. M., & James Cleaves, H. (2010). Catalytic peptide hydrolysis by mineral surface: Implications for prebiotic chemistry. Geochimica et Cosmochimica Acta, 74(20), 5852–5861. doi:10.1016/j.gca.2010.07.009
-
Parikh, S. J., Kubicki, J. D., Jonsson, C. M., Jonsson, C. L., Hazen, R. M., Sverjensky, D. A., & Sparks, D. L. (2011). Evaluating Glutamate and Aspartate Binding Mechanisms to Rutile (α-TiO 2 ) via ATR-FTIR Spectroscopy and Quantum Chemical Calculations. Langmuir, 27(5), 1778–1787. doi:10.1021/la103826p
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Chrisopher Johnson
Collaborator
Donald Sparks
Collaborator
Jennifer Stern
Collaborator
Caroline Jonsson
Postdoc
Katerina Klotchko
Postdoc
Shohei Ohara
Postdoc
Nabil Boctor
Research Staff
Henderson Cleaves
Research Staff
Nami Lee
Graduate Student
-
RELATED OBJECTIVES:
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules