2010 Annual Science Report
 Pennsylvania State University
Reporting | SEP 2009 – AUG 2010
Pennsylvania State University
Reporting | SEP 2009 – AUG 2010
Developing New Biosignatures
Project Summary
The development and experimental testing of potential indicators of life is essential for providing a critical scientific basis for the exploration of life in the cosmos. In microbial cultures, potential new biosignatures can be found among isotopic ratios, elemental compositions, and chemical changes to the growth media. Additionally, life can be detected and investigated in natural systems by directing cutting-edge instrumentation towards the investigation of microbial cells, microbial fossils, and microbial geochemical products. Our efforts are focused on creating innovative approaches for the analyses of cells and other organic material, finding ways in which metal abundances and isotope systems reflect life, and developing creative approaches for using environmental DNA to study present and past life.
Project Progress
Subproject: Raman imaging of kerogen and microfossils as a tool for Astrobiology
Fossil preservation in Recent sulfates:
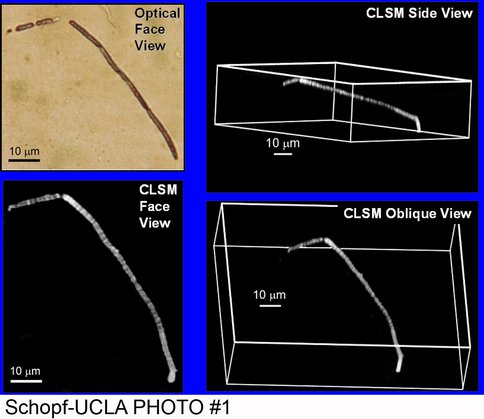
By use of optical microscopy, confocal laser scanning microscopy, and Raman spectroscopy, we have recently discovered and documented the presence of filamentous and coccoidal fossil microorganisms permineralized in Recent gypsum deposits of Baja, Mexico. Coupled with our discovery of diverse gypsum-permineralized microscopic fossils in Miocene (Messinian, ~6-Ma-old) deposits of northern Italy and our studies of Permian sulfate deposits of Texas and New Mexico, this discovery, carried out in collaboration with J.D. Farmer (Arizona State University), has obvious relevance to the search for evidence of past life on Mars where deposits of sulfate, including gypsum, are abundant and widespread.
Optical photomicrograph and three confocal laser scanning micrographs of a cellular microbial filament permineralized in a Recent gypsum deposit of Baja, Mexico.
Microbial biosignatures in Icelandic hot springs:
Optical microscopy, confocal laser scanning microscopy, and Raman spectroscopy have been used to identify microbial biosignatures in and associated with Icelandic hot springs deposits. A manuscript reporting results of this work, with Charles Cockell (a co-PI of the PennState NAI team) as lead author, is currently in preparation.
Possible fungi in Cambrian paleosols:
Using specimens provided by L. Kump and L. Horodyskij (PennState NAI team),our studies by optical microscopy, confocal laser scanning microscopy, and Raman spectroscopy have established the presence of organic filaments, possibly fungi, in samples of the Middle Cambrian (>510 Ma-old), clay-rich Elk Point paleosol (fossilized soil horizon) of Iowa. A manuscript reporting results of this work, with Lev Horodyskij as lead author, has been submitted for publication.
Fossil preservation in Miocene and Permian sulfates:
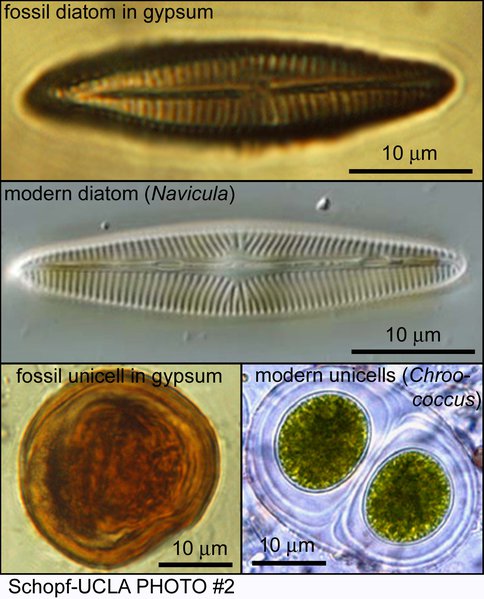
By use of optical microscopy, confocal laser scanning microscopy, and Raman spectroscopy, we have recently discovered and documented the presence of abundant and diverse benthic and planktonic microorganisms (e.g., filamentous and coccoidal cyanobacteria, spheroidal green algae, pennate diatoms, etc.) permineralized in Miocene (Messinian, ~6-Ma-old) gypsum deposits of northern Italy. To extend these studies, we have recently completed detailed fieldwork and sample collection in Permian sulfate deposits of West Texas and southeastern New Mexico. These studies, carried out in collaboration with J. Farmer (Arizona State University), coupled with our recent discovery of gypsum-permineralized microorganisms in Recent sulfate deposits, have obvious relevance to the search for evidence of past life on Mars, where deposits of sulfate, including gypsum are abundant and widespread.
Optical photomicrographs of a diatom and a sheath-enclosed unicell, permineralized in Miocene gypsum from Italy, compared with their modern counterparts.
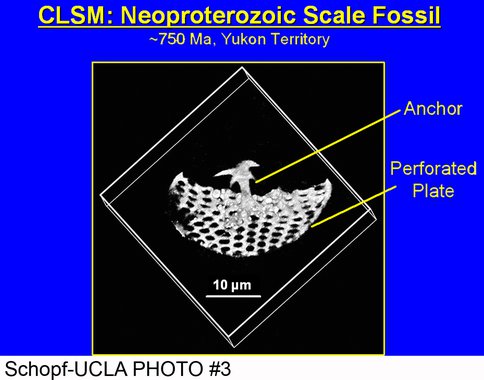
Apatite-biomineralized Neoproterozoic protists:
Using specimens provided by N. J. Butterfield (Cambridge University) and P.A. Cohen (Harvard University), our studies by optical microscopy, confocal laser scanning electron microscopy, and Raman spectroscopy show that ~775-Ma-old protistan scale fossils (from Yukon Territory, Canada) were originally composed of apatite — a surprising discovery, given that the scales of extant protists are most commonly silica or calcite. The Yukon Territory fossils, the earliest example of biomineralization now known from the geological record, have recently been reported by our group in Geology Today, with a more detailed manuscript reporting these findings currently in preparation.
A confocal laser scanning micrograph of a Neoproterozoic scale fossil, composed of apatite and permineralized in chert, the oldest evidence of biomineralization now known from the geological record.
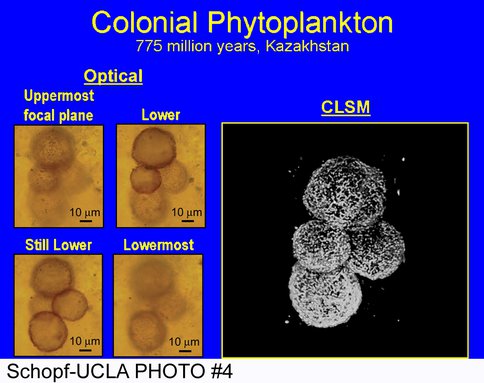
Chert-permineralized Neoproterozoic microbiotas of Kazakhstan:
Two major manuscripts have recently been published that (1) detail the taxonomy and (2) document the usefulness of confocal laser scanning microcopy and Raman spectroscopy for analysis of an exceptionally well-preserved microbial biota chert-permineralized in the Neoproterozoic Chichkan Formation of South Kazakhstan. For studies of Mars rocks, the latter paper, in particular, is especially important since it illustrates the fine-structural and molecular-structural information about fossil microscopic organisms that can be provided by these two non-intrusive, non-destructive analytical techniques. To extend this work — of relevance to the search for past life on Mars, given the well-documented occurrence of siliceous units, including probable hot springs deposits, on the planetary surface — we are currently investigating two additional chert-permineralized Cambrian-age microbiotas of Kazakhstan.
A four-celled microbial colony, permineralized in Neoproterozoic chert from southern Kazakhstan, shown by optical microscopy in four images at increasing focal depths (left) that is decidedly better depicted in a single confocal laser scanning micrograph (right).
Subproject: Weathering and inorganic chemical biosignatures
Basalt weathering
Brantley’s NASA-funded laboratory research targeting dissolution of rocks and minerals in controlled solution chemistries in the laboratory has shown that Fe, Al, P, Cu, Y, and the rare earth elements can be thought of as candidate organomarkers that document the effects of organic molecules in weathered rocks. We observed that the extent of release of Fe and P from basalt in laboratory experiments is enhanced in the presence of citrate as compared to organic-free conditions. We have compiled new data from a laboratory column experiment wherein we measured dissolution rates of basalt with and without organic ligands over six years. Release rates of Si, Mg, Ca and Na from the basalt decreased over the six years; citrate enhanced release by ~5-6x compared to controls with no ligand. We are in the process of compiling similar data from granite-containing columns from the same experiment, and plan to conduct chemical and mineralogical analysis of the solids to assess “organomarkers” of weathering as documented in the lab.
Svalbard weathering and microbial community analysis
As part of AMASE (Arctic Mars Analogue Svalbard Expedition, led by A. Steele), we studied olivine- and glass-containing basaltic rocks from Sverrefjell volcano on Svalbard, Norway, to determine weathering characteristics, and also buried olivine and glass samples at selected locations for one year. These studies were undertaken in an effort to learn more about the presence and duration of water on Mars through comparison of olivine and glass dissolution and persistence in a similar environment. The results of these analyses are reported in Hausrath et al. (2008). Examination of buried samples indicated the potential contribution of biotic weathering processes, e.g. surface features including tube-shaped etchings, decreased Mg/Si ratios, and likely microorganisms and fungal hyphae. These results are consistent with the presence of oxalic acid measured in samples from vegetated sites (Hausrath et al., 2008). Further studies are now underway to characterize the microbial communities present at various sites in Svalbard where some of the samples were obtained, to aid in understanding potential biological contributions to weathering and trace element cycling in this Arctic ecosystem, and to determine their relevance to potential biosignatures on Earth and on Mars.
Microorganisms were present at all locations sampled, with total cell numbers ranging from 108 – 109 cells/g dry soil mass. However, culturable heterotrophic cell numbers were several orders of magnitude lower. At some sites, cell numbers decreased with depth, while at other sites cell numbers remained similar at all depths sampled. Results of iron-related bacterial activity reaction tests (IRB-BART) indicated the likely presence of iron-related bacteria at all sites at > 10 cm depth, and at a vegetated burial site. In addition, anaerobic microorganisms are indicated at all sites at various depths. The highest total and heterotrophic cell counts were observed for the sample from the highest measured porewater acetate concentration. In addition, an inverse relationship appears to exist between the Fe/Si ratio on surfaces of buried samples, as measured by XPS, and the porewater concentrations of Fe and Al. This observation suggests that organic acids are helping to solubilize Fe and Al associated with the mineral surface.
DNA has been extracted from aseptically collected samples from various depths and burial sites. The DNA samples are currently undergoing 454 sequencing with barcoded 16S rDNA primers, with the goal of correlating microbial community composition and metabolic functions with mineralogical and weathering data in this Mars analogue environment.
Biogeochemical cycling of copper
Extremophilic microorganisms, living under conditions such as extremely high or low temperatures, high pressures, high salinity, or low pH and high metal concentrations, are believed to exhibit ancient metabolisms, which were required for the relatively harsh conditions of early earth. Work led by former grad student Bryn Kimball, now a PhD scientist at the USGS, investigated using the mineral jarosite as a biosignature of microbial Fe-oxidation. We grew Acidithiobacillus ferrooxidans, an Fe- and S-oxidizing Proteobacterium, in an FeSO4-based medium at pH 2 and 22 ± 2°C. Over the course of 8 days, precipitates that formed in the presence of the bacteria became increasingly crystalline based on micro-X-ray diffraction analysis. These precipitates were jarosite. In comparison, in parallel abiotic experiments, precipitates that formed were amorphous. We conclude that under the same conditions, biogenic jarosite forms at a faster rate than abiogenic jarosite; thus, in systems that are open with respect to fluid flow, jarosite may be a biosignature.
Subproject: Research Highlights: Intermolecular Isotopic Patterns
Stable isotopic relationships both among different compounds from the same organism and between biomarkers and the biomass composition form the bases for interpretation of these molecular signatures in ancient sediments. Observed patterns in isotopic fractionation associated with biosynthetic pathways reflect both the inherent mass sensitivity of the rate-limiting step (usually an enzymatic reaction) as well as the relative flows of material to different biochemical fates. The magnitude of environmental sensitivity of isotopic fractionation between biomass and molecules are not well known. We have been investigating isotopic patterns in lipids from vascular plants (13C/12C) and in pigments, including scytonomin, in cyanobacteria (15N/14N and 13C/12C).
Highlights:
1) In contrast to previous (and widely employed) reports in the literature (Chikarishi et al., 2004; Monson and Hayes, 1980; DeNiro and Epstein, 1977), and especially in literature for microbial life (reviewed by Hayes, 2002), we consistently find that both MEP and MVA-derived terpenoid compounds in the leaves of higher plants (C3, trees) exhibit minimal fractionation relative to biomass (~ ±1 permil). (Diefendorf Ph.D. thesis; manuscript in preparation)
2) We are the first to observed scytonemin (a marker for cyanobacteria) in ancient sediments. Our findings of this biomarker in pelagic Holocene Black Sea sediments has prompted an investigation of potential sources for this compound, which is typically abundant in mats or other environments with high UV light exposure. Isotopic patterns in Black Sea samples, modern environments and cultures suggest source might include N2-fixing organism (15N) in a soil crusts or coastal mat habitat (13C), although research is continuing. (Fulton Ph.D. thesis, manuscript in preparation).
We are investigating a method for isolation and isotopic analysis of F430, the heme compound in methanogens (and related anaerobic methane oxidizers). We anticipate having results to share at the all-hands meeting. This work is being conducted by Jamey Fulton, and is in collaboration with Victoria Orphan and Rich Pancost.
Subproject: Abiotic production of organic material
Graduate student Karen Smith and PI Christopher H. House are working with Jason Dworkin on the fate of nitrogen-containing heterocyles in prebiotic complex organic mixtures. The work is last year provided a view of the reactivity of several different compounds with the products of an electric discharge. Two heterocycles were found to be reactive and the products forms have been investigated using a suite of analytical tools.
Subproject: Using Methanosarcina to develop new biosignatures (methyl-sulfides and polyphosphate)
Methyl sulfide production
The production of methyl sulfide during growth of methanogens with CO (Appl. Environ. Microbiol. (2008) 74: 540) and a role for methyl sulfide during anaerobic methane oxidation (Environ. Microbiol. (2008) 10: 162) has been postulated to be of biogeochemical importance with potential for development of new biosignatures. The enzymology of Methanosarcina acetivorans is being investigated to better understand the fundamental physiology of the metabolism of methyl sulfides by methanogens; specifically, enzymes proposed in the literature to catalyze the production and/or utilization of the methyl sulfides.
One of three homologous genes (MA4384) from M. acetivorans postulated to react with methyl sulfides based on genetic analyses (Arch. Microbiol. (2007) 188: 463; Mol. Microbiol. (2009) 74: 227) was deleted and growth characterized. The mutant showed impaired growth relative to wild-type when cultured on limiting amounts of CO in contrast to that published for growth with greater concentrations. The results suggest a role for MA4384 during growth on CO in contrast to previously published conclusions. The enzyme produced in Escherichia coli was purified and characterized. Dimethylsulfide was a poor methyl donor relative to methyl-tetrahydromethanopterin (Me-THMPt) and the enzyme had robust Me-THMPt:coenzyme M methyl transferase activity, results consistent with a soluble Me-THMPt:coenzyme M methyl transferase in addition to the membrane-bound enzyme complex.
Poly-phosphate production
Long chain poly-P is ubiquitous to microbial life on Earth (Proc. Natl. Acad. Sci. USA (2004) 101:16085), although few investigations have been reported in species from the Archaea domain. A role for poly-P in stress response has been suggested for the methane-producing archaeon Methanosarcina acetivorans (J. Proteome Res. (2007) 6:759). The probable ancient origin and widespread occurrence in diverse microbes, coupled with the role in coping with extreme environments, identifies poly-P as a facile biosignature for exploration of extraterrestrial life. We are developing poly-P for use as a biosignature by investigating poly-P metabolism in the domain Archaea with M. acetivorans for which the genome is annotated with genes encoding two putative poly-P kinases (Ppk1 and Ppk2) catalyzing synthesis of poly-P.
In order to determine the role of poly-P, a mutant of M. acetivorans deleted of MA2391 encoding Ppk2 was attempted; however, mutants could not be obtained consistent with an essential function for this gene. Mutant strains were constructed in which both poly-P kinase genes are under control of a tetracycline sensitive promoter that will allow controlled expression to further evaluate the physiological functions.
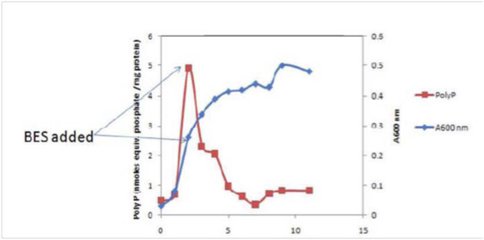
A role for poly-P in supporting growth of M. acetivorans was investigated with methanol-grown cells. Inhibition of methanogenesis with 2-bromoethane sulfonate (BES) failed to inhibit growth and poly-P levels decreased (please see figure), a result suggesting poly-P functions to support growth in the absence of exogenous energy sources.
Inhibition of methanogenesis with 2-bromoethane sulfonate (BES) failed to inhibit growth and poly-P levels decreased (please see figure), a result suggesting poly-P functions to support growth in the absence of exogenous energy sources.
Subproject: Developing Secondary Ion Mass Spectrometry (SIMS) as a tool for Astrobiology
Halogenated probes and SIMS
Efforts to further extend the utility of secondary ion mass spectrometry (SIMS and nanoSIMS), to the study of extant microorganisms and their biosignatures over the past year have included the testing and optimization of halogenated oligonucleotide probes (fluorinated 16S rRNA oligonucleotide probes) and the use of quantum dots, specifically Se ions, for combined whole-cell immunofluorescence and nanoSIMS ion imaging of expressed proteins.
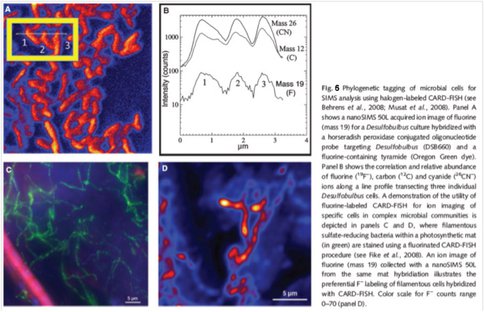
Experiments using a modified halogenated CARD-FISH assay to stain microorganisms in sediments and microbial mats has yielded mixed results, possibly due to heterogeneous background levels of fluorine in these complex environmental samples. The figure below provides an example of successful F- detection in pure cultures and a microbial mat (from Orphan and House, 2009). Additional efforts to optimize this assay are ongoing.
Quantum Dots: imaging and nanoSIMS detection of expressed proteins in environmental microorganisms
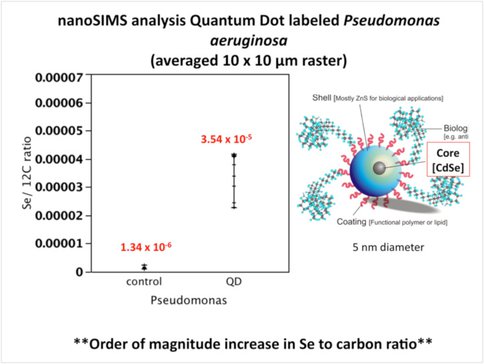
To explore the potential use of quantum dots correlate fluorescence protein detection and SIMS analysis, we are conducting a series of nanoSIMS experiments using quantum dot labeled Pseudomonas in collaboration with P. Holden at the University of California Santa Barbara. Quantum dots frequently contain a cadmium, selenium, zinc sulfide core and may be amenable to detection by SIMS through the detection of select metal ions, like selenium. NanoSIMS analysis of Pseudomonas cultures labeled with quantum dots contained at least 25 times greater selenium/ carbon ratio relative to unlabeled control cells. This illustrates the possibility of using Se ions as a means of intracellular localization of qDot labeled antibodies or DNA probes for microbial cells in combination with isotope labeling experiments using the nanoSIMS (and possibly the 1270 instrument equipped with a Ga+ primary ion beam at UCLA).
Subproject: Isotopic biosignatures in minerals
The generation and recording of isotopic biosignatures in minerals is the general focus of this subproject. We are specifically interested in determining if microbes interact with their surrounding environment in a manner that affects the isotopic composition of minerals. We begin this investigation last year, by collecting gypsum samples from sulfidic caves and measuring the calcium and sulfur isotopic composition of these samples. This year we initiated abiotic experiments aimed at elucidating the abiotic isotope effects, thus serving as context for evaluting the previous measurements.
In the near future, we will be analyzing the Ca isotopic composition of our experimental products. We anticipate this will occur in SPR2011 and SUMM2011.
In YR 2 of the PSARC proposal, we have accomplished the following:
- Designed and build four experimental reactors
- Includes rebuilding a Thermo temperature controlled water bath
- Calibrated the Ca ISE probe for real-time measurements of Ca concentrations
- We use this to calculate precipitation rates. To date, we have completed ten experiments aimed at helping us to understand the system.
- Imaged two of the experiments by reflected light microscopy (Figs. 8) and SEM.
- The size and morphology of the precipitated crystals closely resemble the gypsum toothpaste and needles that we sampled last year in the Frasassi caves.
- Completed x-ray diffraction measurements of the two imaged experiments.
- The data show that the crystals are pure gypsum at the percent level.
- Calculated activity coefficients and saturation states for gypsum and calcite in our experiments using Pitzer formalism (not shown).
- Results indicate that we are not saturated with respect to calcite and should precipitate gypsum. At the ionic strengths of our experiments, we are able to achieve saturation states (Ω) from ~1.1 to 6.3. One goal of our experiments is to evaluate the effect of saturation state on Ca isotope fractionation in gypsum.
Reflected light microscopy of precipitated gypsum crystals. (Left) Small nanometer-sized gypsum crystals at 400X magnification. (Right) Micron-sized gypsum crystals at 400X magnification.
Subproject: DNA as a biosignature
Ancient DNA and DNA preservation
Dr. Beth Shapiro (PSU) has continued to work on fossil DNA and its preservation with two papers published during the reporting period and a couple more in press.
Metagenomics as a tool for Astrobiology
Marine subsurface sediments are low to extremely low in cell numbers, and consequently, in amount of DNA available. When DNA concentrations are very low, amplification must be done prior to sequencing and other analyses. Past methods of amplification have proven problematic due to the in ability to obtain a reliable negative control (methods produced product no matter what). We are developing a PCR based genomic DNA amplification method to address that specific problem. In developing Random Amplicon Metagenomic PCR (RAMP), we have experimented with various parameters involved in the amplification reaction, such as annealing temperatures, number of PCR cycles, different DNA polymerases, primer concentrations, etc. After optimization of these parameters, we tested the method to an organism whose complete genome has been sequenced (E. coli) in order to assess biases in the amplification process. In addition, we have applied the method to marine subsurface samples from several locations. We are currently working on analyzing and summarizing my results for a publication.
In a realated project, past and current DNA studies of marine subsurface microbiology have shown that within the “rare biosphere” (that is, unique sequences that make up only a very small portion of the community), there is evidence for phototrophic organisms. This evidence is in the form of 16S DNA from cyanobacteria, as well as matches to genes involved in photosynthesis. This research focuses on further showing the possibility for persistence and survival of phototrophic organisms in deeply buried marine sediments after presumed delivery from the ocean surface. To study this possibility, we have attempted to culture organisms from sediment samples in phototrophic media. After about 6 months, evidence of growth was obtained in some of the samples, which were subjected to DNA extraction and 16S PCR amplification. These samples were just sequenced and we are currently working on analyzing the results for a publication.
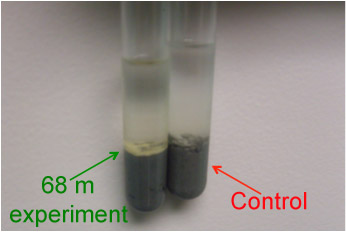
Tubes showing the results of six-month incubations under light at 4°C with marine sediment recovered from 68 meters below the seafloor.
Publications
-
MOCZYDŁOWSKA, M., Schopf, J. W., & Willman, S. (2009). Micro- and nano-scale ultrastructure of cell walls in Cryogenian microfossils: revealing their biological affinity. Lethaia, 43(2), 129–136. doi:10.1111/j.1502-3931.2009.00175.x
-
Barnett, R., Shapiro, B., Barnes, I., Ho, S. Y. W., Burger, J., Yamaguchi, N., … Cooper, A. (2009). Phylogeography of lions ( Panthera leo ssp.) reveals three distinct taxa and a late Pleistocene reduction in genetic diversity. Molecular Ecology, 18(8), 1668–1677. doi:10.1111/j.1365-294×.2009.04134.x
-
Bunce, M., Worthy, T. H., Phillips, M. J., Holdaway, R. N., Willerslev, E., Haile, J., … Cooper, A. (2009). The evolutionary history of the extinct ratite moa and New Zealand Neogene paleogeography. Proceedings of the National Academy of Sciences, 106(49), 20646–20651. doi:10.1073/pnas.0906660106
-
Campos, P. F., Willerslev, E., Sher, A., Orlando, L., Axelsson, E., Tikhonov, A., … Gilbert, M. T. P. (2010). Ancient DNA analyses exclude humans as the driving force behind late Pleistocene musk ox (Ovibos moschatus) population dynamics. Proceedings of the National Academy of Sciences, 107(12), 5675–5680. doi:10.1073/pnas.0907189107
-
Collins, M. J., Penkman, K. E. H., Rohland, N., Shapiro, B., Dobberstein, R. C., Ritz-Timme, S., & Hofreiter, M. (2009). Is amino acid racemization a useful tool for screening for ancient DNA in bone?. Proceedings of the Royal Society B: Biological Sciences, 276(1669), 2971–2977. doi:10.1098/rspb.2009.0563
-
Czaja, A. D., Johnson, C. M., Beard, B. L., Eigenbrode, J. L., Freeman, K. H., & Yamaguchi, K. E. (2010). Iron and carbon isotope evidence for ecosystem and environmental diversity in the ∼2.7 to 2.5Ga Hamersley Province, Western Australia. Earth and Planetary Science Letters, 292(1-2), 170–180. doi:10.1016/j.epsl.2010.01.032
-
Dekas, A. E., & Orphan, V. J. (2011). Identification of Diazotrophic Microorganisms in Marine Sediment via Fluorescence In Situ Hybridization Coupled to Nanoscale Secondary Ion Mass Spectrometry (FISH-NanoSIMS). Methods in Enzymology, None, 281–305. doi:10.1016/b978-0-12-381294-0.00012-2
-
Diefendorf, A. F., Mueller, K. E., Wing, S. L., Koch, P. L., & Freeman, K. H. (2010). Global patterns in leaf 13C discrimination and implications for studies of past and future climate. Proceedings of the National Academy of Sciences, 107(13), 5738–5743. doi:10.1073/pnas.0910513107
-
Ferry, J. G. (2010). CO in methanogenesis. Annals of Microbiology, 60(1), 1–12. doi:10.1007/s13213-009-0008-5
-
Ferry, J. G. (2010). How to Make a Living by Exhaling Methane. Annu. Rev. Microbiol., 64(1), 453–473. doi:10.1146/annurev.micro.112408.134051
-
Ferry, J. G. (2010). The chemical biology of methanogenesis. Planetary and Space Science, 58(14-15), 1775–1783. doi:10.1016/j.pss.2010.08.014
-
Firth, C., Kitchen, A., Shapiro, B., Suchard, M. A., Holmes, E. C., & Rambaut, A. (2010). Using Time-Structured Data to Estimate Evolutionary Rates of Double-Stranded DNA Viruses. Molecular Biology and Evolution, 27(9), 2038–2051. doi:10.1093/molbev/msq088
-
Hausrath, E. M., Neaman, A., & Brantley, S. L. (2009). Elemental release rates from dissolving basalt and granite with and without organic ligands. American Journal of Science, 309(8), 633–660. doi:10.2475/08.2009.01
-
Ho, S. Y. W., Lanfear, R., Phillips, M. J., Barnes, I., Thomas, J. A., Kolokotronis, S-O., & Shapiro, B. (2011). Bayesian Estimation of Substitution Rates from Ancient DNA Sequences with Low Information Content. Systematic Biology, 60(3), 366–375. doi:10.1093/sysbio/syq099
-
Kuhn, T. S., McFarlane, K. A., Groves, P., Mooers, A. Ø., & Shapiro, B. (2010). Modern and ancient DNA reveal recent partial replacement of caribou in the southwest Yukon. Molecular Ecology, 19(7), 1312–1323. doi:10.1111/j.1365-294×.2010.04565.x
-
Lal, D., Schopf, J. W., Abbott, P. L., Vacher, L., Jull, A. J. T., & McHargue, L. (2010). Nuclear, chemical and biological characterization of formation histories of ironstones from several sites in Southern California: Dominant role of bacterial activity. Earth and Planetary Science Letters, 296(3-4), 227–234. doi:10.1016/j.epsl.2010.05.002
-
McInerney, F. A., Helliker, B. R., & Freeman, K. H. (2011). Hydrogen isotope ratios of leaf wax n-alkanes in grasses are insensitive to transpiration. Geochimica et Cosmochimica Acta, 75(2), 541–554. doi:10.1016/j.gca.2010.10.022
-
Mueller, K. E., Diefendorf, A. F., Freeman, K. H., & Eissenstat, D. M. (2010). Appraising the roles of nutrient availability, global change, and functional traits during the angiosperm rise to dominance. Ecology Letters, 13(5), E1–E6. doi:10.1111/j.1461-0248.2010.01455.x
-
Polissar, P. J., & Freeman, K. H. (2010). Effects of aridity and vegetation on plant-wax δD in modern lake sediments. Geochimica et Cosmochimica Acta, 74(20), 5785–5797. doi:10.1016/j.gca.2010.06.018
-
Polissar, P. J., Freeman, K. H., Rowley, D. B., McInerney, F. A., & Currie, B. S. (2009). Paleoaltimetry of the Tibetan Plateau from D/H ratios of lipid biomarkers. Earth and Planetary Science Letters, 287(1-2), 64–76. doi:10.1016/j.epsl.2009.07.037
-
Polissar, P. J., Fulton, J. M., Junium, C. K., Turich, C. C., & Freeman, K. H. (2009). Measurement of 13 C and 15 N Isotopic Composition on Nanomolar Quantities of C and N. Anal. Chem., 81(2), 755–763. doi:10.1021/ac801370c
-
Rambaut, A., Ho, S. Y. W., Drummond, A. J., & Shapiro, B. (2008). Accommodating the Effect of Ancient DNA Damage on Inferences of Demographic Histories. Molecular Biology and Evolution, 26(2), 245–248. doi:10.1093/molbev/msn256
-
Rijsdijk, K. F., Hume, J. P., Bunnik, F., Florens, F. B. V., Baider, C., Shapiro, B., … Gittenberger, E. (2009). Mid-Holocene vertebrate bone Concentration-Lagerstätte on oceanic island Mauritius provides a window into the ecosystem of the dodo (Raphus cucullatus). Quaternary Science Reviews, 28(1-2), 14–24. doi:10.1016/j.quascirev.2008.09.018
-
Schopf, J. W., & Kudryavtsev, A. B. (2010). A renaissance in studies of ancient life. Geology Today, 26(4), 140–145. doi:10.1111/j.1365-2451.2010.00760.x
-
Schopf, J. W., Kudryavtsev, A. B., & Sergeev, V. N. (2010). Confocal Laser Scanning Microscopy and Raman Imagery of the Late Neoproterozoic Chichkan Microbiota of South Kazakhstan. Journal of Paleontology, 84(3), 402–416. doi:10.1666/09-134.1
-
Schopf, J. W., Kudryavtsev, A. B., Sugitani, K., & Walter, M. R. (2010). Precambrian microbe-like pseudofossils: A promising solution to the problem. Precambrian Research, 179(1-4), 191–205. doi:10.1016/j.precamres.2010.03.003
-
Schouten, S., Hopmans, E. C., Van Der Meer, J., Mets, A., Bard, E., Bianchi, T. S., … Sinninghe Damsté, J. S. (2009). An interlaboratory study of TEX 86 and BIT analysis using high-performance liquid chromatography-mass spectrometry. Geochem. Geophys. Geosyst., 10(3), n/a–n/a. doi:10.1029/2008gc002221
-
Sergeev, V. N., & William Schopf, J. (2010). Taxonomy, Paleoecology and Biostratigraphy of the Late Neoproterozoic Chichkan Microbiota of South Kazakhstan: The Marine Biosphere on the Eve of Metazoan Radiation. Journal of Paleontology, 84(3), 363–401. doi:10.1666/09-133.1
-
Taylor, P. D., Vinn, O., Kudryavtsev, A., & William Schopf, J. (2010). Raman spectroscopic study of the mineral composition of cirratulid tubes (Annelida, Polychaeta). Journal of Structural Biology, 171(3), 402–405. doi:10.1016/j.jsb.2010.05.010
-
Thomas, B., Freeman, K. H., & Arthur, M. A. (2009). Intramolecular carbon isotopic analysis of acetic acid by direct injection of aqueous solution. Organic Geochemistry, 40(2), 195–200. doi:10.1016/j.orggeochem.2008.10.011
-
Weinstock, J., Shapiro, B., Prieto, A., Marín, J. C., González, B. A., Gilbert, M. T. P., & Willerslev, E. (2009). The Late Pleistocene distribution of vicuñas (Vicugna vicugna) and the “extinction” of the gracile llama (“Lama gracilis”): New molecular data. Quaternary Science Reviews, 28(15-16), 1369–1373. doi:10.1016/j.quascirev.2009.03.008
-
William Schopf, J. (2010). The paleobiological record of photosynthesis. Photosynthesis Research, 107(1), 87–101. doi:10.1007/s11120-010-9577-1
-
Zazula, G. D., MacKay, G., Andrews, T. D., Shapiro, B., Letts, B., & Brock, F. (2009). A late Pleistocene steppe bison (Bison priscus) partial carcass from Tsiigehtchic, Northwest Territories, Canada. Quaternary Science Reviews, 28(25-26), 2734–2742. doi:10.1016/j.quascirev.2009.06.012
- Narbonne, G.M., Schopf, J.W. & Walter, M.R. (2010). Hans J. Hofmann (1936-2010). Geolog, 39(2): 13-14.
- Nasdala, L., Beyssac, O., Schopf, J.W. & Bleisteiner, B. (2010, In Press). Application of Raman-based images in the Earth sciences. In: Zoubir, A. (Eds.). Raman Imaging. Amsterdam: Springer.
- Richards, M., Shapiro, B., Ditchfield, P. & Cooper, A. (2010, In Press). Bison bone collagen isotopic values track climate fluctuations and vegetation change in Late Pleistocene and Early Holocene Northern Eurasia and North America. Geol. J.
- Schopf, J.W. & Kudryavtsev, A.B. (2010, In Press). Confocal laser scanning microscopy and Raman (and fluorescence) spectroscopic imagery of permineralized Cambrian and Neoproterozoic fossils. In: M. Laflamme, S.D.a.J.S. (Eds.). Quantifying the Early Evolution of Life: Numerical and Technological Approaches to the Study of Fossils and Ancient Ecosystems. Amsterdam: Springer.
- Schopf, J.W. (2010, In Press). The fossil record of cyanobacteria. In: Whitton, B.A. (Eds.). Ecology of Cyanobacteria II. Berlin Heidelberg New York: Springer.
- Schopf, J.W., Kudryavtsev, A.B., Tripathi, B. & Czaja, A.D. (2010, In Press). Three-dimensional Morphological (CLSM) and Chemical (Raman) Imagery of Cellularly Mineralized Fossils. In: Bottjer, P.A.A.a.D.J. (Eds.). Taphonomy: Process and Bias Through Time. Amsterdam: Springer.
- Turich, C.H. & Freeman, K.H. (2010, In Press). Archaea lipids record transition from marine to hypersaline conditions. Organic Geochemistry.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Nicholas Butterfield
Collaborator
Charles Cockell
Collaborator
George Cody
Collaborator
Phoebe Cohen
Collaborator
Andrew Czaja
Collaborator
Jason Dworkin
Collaborator
Jack Farmer
Collaborator
Duane Froese
Collaborator
Libby Hausrath
Collaborator
Francis Macdonald
Collaborator
Vladimir Sergeev
Collaborator
Yongchun Tang
Collaborator
Maya Bhatt
Postdoc
Suharti Suharti
Postdoc
Venkata Vepachedu
Postdoc
John Cantolina
Research Staff
Anatoliy Kudryavtsev
Research Staff
Laura Liermann
Research Staff
Dennis Walizer
Research Staff
Yumiko Watanabe
Research Staff
Zhidan Zhang
Research Staff
Michael Avery
Graduate Student
Leah Brandt
Graduate Student
Anne Dekas
Graduate Student
Ian Foster
Graduate Student
Khadouja Harouaka
Graduate Student
Lev Horodyskyj
Graduate Student
Bryn Kimball
Graduate Student
Lexan Lhu
Graduate Student
Mandi Martino
Graduate Student
Karen Smith
Graduate Student
Tiffany Yesavage
Graduate Student
Danielle Gruen
Undergraduate Student
Kati Shea
Undergraduate Student
-
RELATED OBJECTIVES:
Objective 2.1
Mars exploration.
Objective 2.2
Outer Solar System exploration
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules
Objective 3.4
Origins of cellularity and protobiological systems
Objective 4.1
Earth's early biosphere.
Objective 5.2
Co-evolution of microbial communities
Objective 5.3
Biochemical adaptation to extreme environments
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems