2009 Annual Science Report
 VPL at University of Washington
Reporting | JUL 2008 – AUG 2009
VPL at University of Washington
Reporting | JUL 2008 – AUG 2009
Thermodynamic Efficiency of Electron-Transfer Reactions in the Chlorophyll D-Containing Cyanobacterium, Acharyochloris Marina
Project Summary
Photosynthesis is the only known process that produces planetary-scale biosignatures – atmospheric oxygen and the color of photosynthetic pigments — and it is expected to be successful on habitable extrasolar planets as well, due to the ubiquity of starlight as an energy source. How might photosynthetic pigments adapt to alternative environments? Could oxygenic photosynthesis occur at much longer wavelengths than the red? This project is approaching these questions by studying a recently discovered cyanobacterium, Acaryochloris marina, which performs oxygenic photosynthesis in environments depleted in visible light but enriched in far-red/near-infrared light. A. marina is the only known organism to have chlorophyll d (Chl d) to use photons in the far-red and near-infrared, whereas all other oxygenic photosynthetic organisms use chlorophyll a (Chl a) to utilize red photons. Whether A. marina is operating more efficiently or less than Chl a-utilizing organisms will indicate what wavelengths are the ultimate limit for oxygenic photosynthesis. We have been conducting lab measurements of energy storage in whole A. marina cells using pulsed, time-resolved photoacoustics (PTRPA, or PA), a laser technique that allows us to control the wavelength, amount, and timing of energy received by a sample of cells.
Project Progress
Photoacoustic measurements of thermodynamic efficiency of photon energy use in Acaryochloris marina (Kiang, Blankenship, Mielke, collaborators at Rockefeller University, City College of New York).
Under funding from the NAI Director’s Discretionary Fund (DDF) 2008, we have been conducting measurements the efficiency of photon energy storage by wavelength in Photosystem I in whole intact cells of the cyanobacterium Acaryochloris marina. A. marina is the only known organism to have chlorophyll d (Chl d) to use photons in the far-red (713-715 nm) and near-infrared (740 m), whereas all other oxygenic photosynthetic organisms use chlorophyll a (Chl a) to absorb at 680 nm and 700 nm. We have obtained preliminary measurements indicating that A. marina may be exhibiting lower efficiencies hence lower quantum yields compared to Chl a-utilizing organisms, i.e. the red wavelengths could be at a fundamental limit for oxygenic photosynthesis and thus constrain what is plausible on extrasolar planets. Confirmation with further measurements continues, after a laser failure in January 2009.
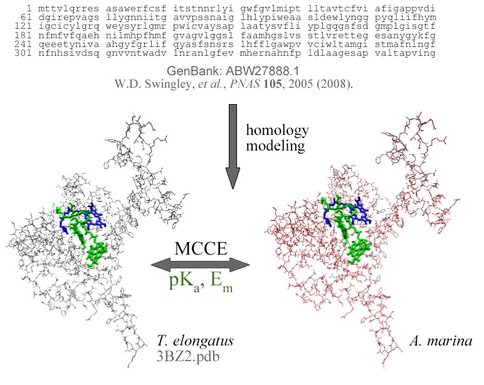
Complementing these lab studies, NAI postdoctoral fellow Steven Mielke is now also using the Multi-Conformer Continuum Electrostatics (MCCE) computer program of a new collaborator, Dr. Marilyn Gunner, at City College of New York to model redox potentials along the electron transfer pathway in Photosystem II with Chl a versus Chl d. This theoretical work will predict, confirm, and/or explain results of the photoacoustic measurements. In October 2009, the project was awarded additional NAI DDF funds to enhance the lab studies with measurements on purified complexes of Photosystems I and II.
Conferences:
Kiang, N.Y., S.P. Mielke, D. Mauzerall, R.E. Blankenship. “Limits of photosynthesis:
non-Earthlike target biosignatures, “Pathways Toward Habitable Planets, Barcelona, Spain, 14-18 September 2009,” oral presentation.
Kiang, N.Y. “Earth remote sensing for the search for life elsewhere in the universe: discerning spectral signatures of photosynthesis on extrasolar planets,” 2nd HyspIRI Science Workshop, Sheraton Hotel, Pasadena, August 11-13, 2009, poster.
Kiang, N.Y. “Astrobiology of Photosynthesis: Color Limits for Life Adapted to Other Stars and Atmospheres,” Kavli Symposium, Beckman Center, University of California, Irvine, November 6-8, 2008, Invited talk.
Mielke, SP, NY Kiang, RE Blankenship, D Mauzerall. “Energy storage of oxygenic photosynthesis in the cyanobacterium, Acaryochloris marina, investigated by photoacoustics,” Eastern Regional Photosynthesis Conference (ERPC), Marine Biological Laboratory, Woods Hole, MA, April 17 – 19, 2009, poster.
Mielke, SP , NY Kiang, RE Blankenship, D Mauzerall. “Energy storage of oxygenic photosynthesis in the cyanobacterium, Acaryochloris marina, investigated by photoacoustics,” Gordon Research Conference – Photosynthesis, Bryant University, Smithfield, RI, June 28 – July 3, 2009, poster.
Mielke, SP , NY Kiang, RE Blankenship, D Mauzerall. “Energy storage of oxygenic photosynthesis in the cyanobacterium, Acaryochloris marina, investigated by photoacoustics,” Weizmann UK Symposium – Light Energy for a Brighter Future, Imperial College and Royal Geographical Society, London, UK, July 6 – 7, 2009, poster.
Mielke. SP. “Photosynthetic Electron Transfer in the Cyanobacterium, Acaryochloris marina,” NAI Executive Council Meeting, Yellowstone National Park, Wyoming, September 10 – 11, 2009, talk.

Pulsed, time-resolved photoacoustic (PTRPA) signals from A. marina intact cells generated by a 1-nanosecond, 730 nm laser pulse at ~0.4 microjoules. The lower (red) trace was obtained in the dark, and the upper (black) trace in the presence of saturating continuous white light. The difference in the amplitudes of the signals represents photosynthetic energy storage; i.e., less heat is released by the unsaturated cells in the dark, because some of the incident energy is stored in the products of photochemistry.
Steps to modeling redox potentials and equilibrium constants in Photosystem II: homology modeling from amino acid sequences from the GenBank database, and then electrostatics modeling with the Multi-Conformer Continuum Electrostatics (MCCE) program, comparing Chl a-utilizing Thermosynechococcus elongatus with Chl d-utilizing Acaryochloris marina.
Publications
- Mielke, S., Kiang, N., Blankenship, R. & Mauzerall, D. (April 17-19, 2009). Energy storage of oxygenic photosynthesis in the cyanobacterium, Acaryochloris marina, investigated by photoacoustics. Regional Photosynthesis Conference (ERPC). Marine Biological Laboratory, Woods Hole, MA.
- Mielke, S., Kiang, N., Blankenship, R. & Mauzerall, D. (July 6-7, 2009). Energy storage of oxygenic photosynthesis in the cyanobacterium, Acaryochloris marina, investigated by photoacoustics. Weizmann UK Symposium – Light Energy for a Brighter Future. Imperial College and Royal Geographical Society, London, UK.
- Mielke, S., Kiang, N., Blankenship, R. & Mauzerall, D. (June 28 – July 3, 2009). Energy storage of oxygenic photosynthesis in the cyanobacterium, Acaryochloris marina, investigated by photoacoustics. Gordon Research Conference – Photosynthesis. Bryant University, Smithfield, RI.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Robert Blankenship
Co-Investigator
David Mauzerall
Co-Investigator
Marilyn Gunner
Collaborator
Steven Mielke
Postdoc
-
RELATED OBJECTIVES:
Objective 3.2
Origins and evolution of functional biomolecules
Objective 4.2
Production of complex life.
Objective 5.1
Environment-dependent, molecular evolution in microorganisms
Objective 5.3
Biochemical adaptation to extreme environments
Objective 6.2
Adaptation and evolution of life beyond Earth
Objective 7.2
Biosignatures to be sought in nearby planetary systems