2009 Annual Science Report
 VPL at University of Washington
Reporting | JUL 2008 – AUG 2009
VPL at University of Washington
Reporting | JUL 2008 – AUG 2009
Postdoctoral Fellow Report: Steven Mielke
Project Summary
This project seeks to resolve the long-wavelength limit of oxygenic photosynthesis in order to constrain the range of extrasolar environments in which spectral signatures of biogenic oxygen might be found, and thereby guide future planet detecting and characterizing observatories.
Project Progress
My research efforts during the first year of my NAI NPP fellowship have sought to address the long-wavelength limit of oxygenic photosynthesis to advance the goals of the VPL task described in detail in the “Thermodynamic Efficiency of Electron-Transfer Reactions in the Chlorophyll d-Containing Bacterium Acharyochloris Marina” project report. These efforts have consisted of: (a) an experimental component conducted in collaboration with the Mauzerall laboratory, Rockefeller University, and (b) a theoretical component, conducted in collaboration with the Gunner laboratory, City College of New York. Both components have focused on a unique cyanobacterium, A. marina, that uses an unusual chlorophyll to perform oxygenic photosynthesis in far-red light environments [1].
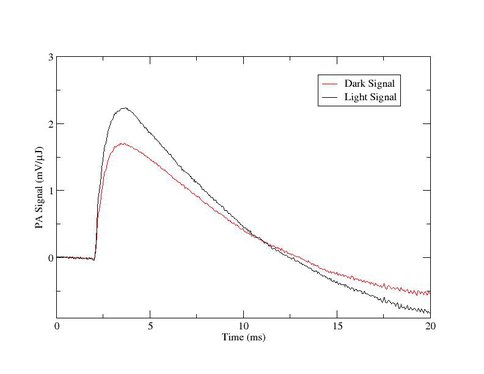
Progress in area (a) has included preliminary photoacoustic measurements [2]—the first obtained from this organism—suggesting the possibility that the efficiency of photochemistry in A. marina is less than that in typical cyanobacteria (see Fig. 1). If confirmed, this result will provide evidence that wavelengths longer than ≈700 nm are fundamentally limiting to oxygenic photochemistry on Earth, which potentially has implications for the range of extrasolar environments in which spectral signatures of biogenic oxygen might be found. In Year 2, I will repeat and refine these measurements; for example, by obtaining photosystem-specific efficiencies using isolated reaction centers prepared in the laboratory of my NPP Co-Advisor, Robert Blankenship, Washington University. This will potentially identify the stage(s) of photochemistry that limit oxygen production. I anticipate at least one publication from this work by the end of Year 2. Support for this effort by the NAI DDF was recently renewed for FY 2009.
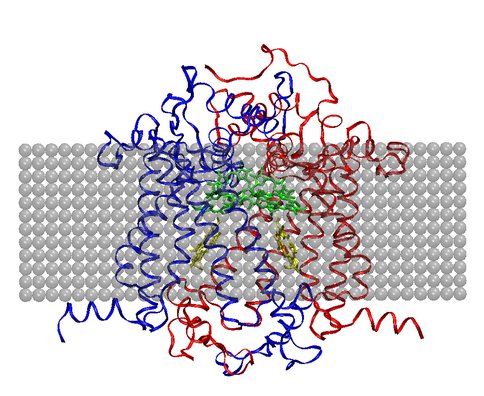
Progress in area (b) has included development of three-dimensional structural models of Photosystem II (PSII) in A. marina (see Fig. 2), and calculation of the midpoint redox potentials of its principal cofactors, as well as of those in the cyanobacterium, T. elongatus, using MCCE [3]. Comparison between the two species indicates that the potentials of their redox cofactors are not significantly different, suggesting that molecular adaptations in PSII of A. marina to accommodate lower photon energies have not significantly affected photochemistry in this photosystem. In Year 2, I will continue this work by expanding and refining the structural models-for example, by incorporating the PSII subunits omitted from the present work-and constraining the redox potential differences needed for efficient electron transfer by numerically solving the relevant coupled rate equations. I anticipate at least one publication from this effort by the end of Year 2.
Figure 1. Photoacoustic signals from A. marina intact cells generated by a 1-ms, 730 nm laser pulse at »0.4 mJ. The lower (red) trace was obtained in the dark, and the upper (black) trace in the presence of saturating continuous white light. The ratio of the difference between the amplitudes to the amplitude of the upper trace gives the thermal efficiency of photochemical energy storage, here »25%.
Figure 1: Photoacoustic signals from A. marina intact cells generated by a 1-μs, 730 nm laser pulse at ≈0.4 μJ. The lower (red) trace was obtained in the dark, and the upper (black) trace in the presence of saturating continuous white light. The ratio of the difference between the amplitudes to the amplitude of the upper trace gives the thermal efficiency of photochemical energy storage, here ≈25%.
Figure 2. D1 (blue) and D2 (red) protein subunits in PSII of A. marina, modeled using SWISS-MODEL (http://swissmodel.expasy.org/workspace/). Corresponding primary and accessory chlorophylls are green, and pheophytins yellow. Gray spheres represent the thylakoid membrane in which the photosystem is embedded.
Figure 2: D1 (blue) and D2 (red) protein subunits in PSII of A. marina, modeled using SWISS-MODEL (http://swissmodel.expasy.org/workspace/). Corresponding primary and accessory chlorophylls are green, and pheophytins yellow. Gray spheres represent the thylakoid membrane in which the photosystem is embedded.
References
[1] A.W.D. Larkum and M. Kühl, Trends in Plant Sci. 10, 355 (2005).
[2] D. Charlebois and D. Mauzerall, Photosynth. Res. 59, 27 (1999).
[3] Y. Song, J. Mao, and M. R. Gunner, J. Comp. Chem. 30, 2231 (2009).
Publications
- Mielke, S., Kiang, N., Blankenship, R. & Mauzerall, D. (April 17-19, 2009). Energy storage of oxygenic photosynthesis in the cyanobacterium, Acaryochloris marina, investigated by photoacoustics. Regional Photosynthesis Conference (ERPC). Marine Biological Laboratory, Woods Hole, MA.
- Mielke, S., Kiang, N., Blankenship, R. & Mauzerall, D. (July 6-7, 2009). Energy storage of oxygenic photosynthesis in the cyanobacterium, Acaryochloris marina, investigated by photoacoustics. Weizmann UK Symposium – Light Energy for a Brighter Future. Imperial College and Royal Geographical Society, London, UK.
- Mielke, S., Kiang, N., Blankenship, R. & Mauzerall, D. (June 28 – July 3, 2009). Energy storage of oxygenic photosynthesis in the cyanobacterium, Acaryochloris marina, investigated by photoacoustics. Gordon Research Conference – Photosynthesis. Bryant University, Smithfield, RI.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Victoria Meadows
Project Investigator
Robert Blankenship
Co-Investigator
Marilyn Gunner
Co-Investigator
Nancy Kiang
Co-Investigator
David Mauzerall
Co-Investigator
-
RELATED OBJECTIVES:
Objective 5.1
Environment-dependent, molecular evolution in microorganisms
Objective 6.1
Effects of environmental changes on microbial ecosystems
Objective 6.2
Adaptation and evolution of life beyond Earth
Objective 7.2
Biosignatures to be sought in nearby planetary systems