2014 Annual Science Report
 University of Wisconsin
Reporting | SEP 2013 – DEC 2014
University of Wisconsin
Reporting | SEP 2013 – DEC 2014
Project 2G: Iron Isotope Fractionations Among Oxide Minerals Under Acidic Conditions
Project Summary
The study of Fe isotope exchange and fractionation between aqueous Fe(II) and goethite was motivated by the inferred acidic environment for early Mars, where iron oxides (i.e. jarosite, goethite) were likely present. We found that the extent of atom exchange positively correlates with increasing pH during interactions between Fe(II)aq and goethite. The decrease in extent of exchange correlates with a decrease in the amount of sorbed Fe(II) to the goethite surface, which strongly suggests that sorbed Fe(II) is the primary catalyst for inducing Fe isotope exchange. The slow rate of isotopic exchange at acidic pH suggests that stable Fe isotope compositions may be resistant to change in acidic aqueous environments, thus leading to preservation of signatures that might contribute to the understanding of ancient Mars paleoenvironments.
Project Progress
Previous experiments on the stable Fe isotope fractionations and exchange kinetics between aqueous Fe(II) and Fe oxides have been conducted at circumneutral pH, yet little work has been done under acidic conditions. In this study, pH was manipulated in order to observe variations in the extent of sorption of Fe(II)aq onto goethite, and its influence on Fe(II)-Fe(III) interactions. We hypothesized that as sorption of Fe(II)aq onto goethite decreased, this would slow isotopic exchange. We did not anticipate significant changes in the equilibrium Fe(II)aq-goethite fractionation factor at low pH because there is little change in the major aqueous Fe(II) species as compared to circumneutral pH. As expected, we observe that sorption correlates with pH. The trajectories of δ56Fe varies at variable pH suggest distinct initial kinetic isotope effects, although finally approaching similar δ56Fe values.
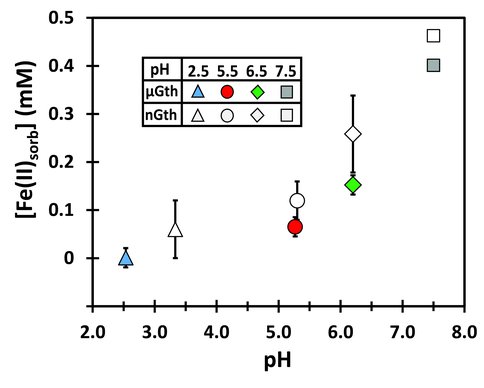
Two sizes of goethite were used. One is termed “nano goethite”, denoted as nGoe, which had a BET surface area of 119 m2g-1. The second is termed “micro goethite”, denoted as µGoe, which had a BET surface area of 43m2g-1. The extent of sorption of Fe(II) to the goethite surface correlated with pH, and higher extents of sorption were observed for nGoe as compared to µGoe, as expected for the contrast in surface area (Figure 1).
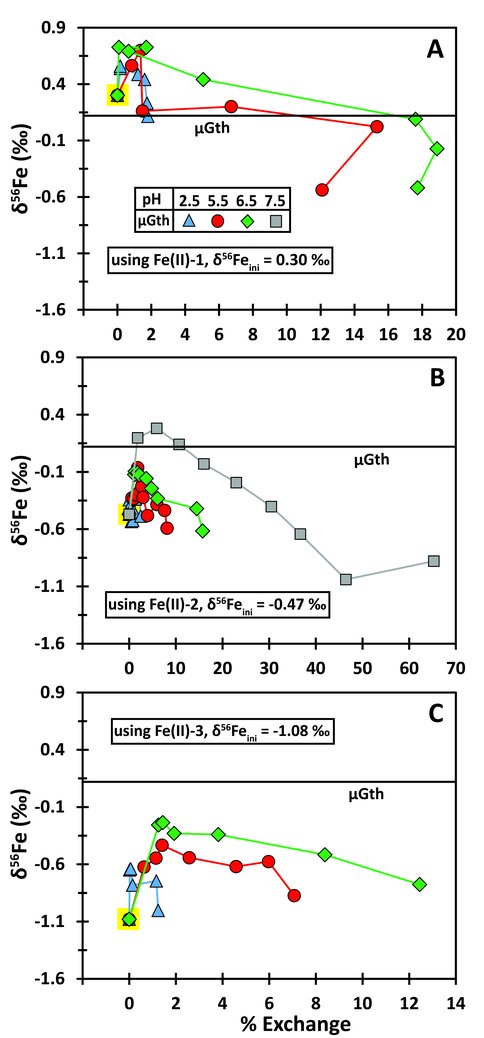
The extent of Fe isotope exchange was monitored using an enriched 57Fe tracer, and changes in the instantaneous Fe isotope fractionation factor was were monitored using the “natural” isotope ratio 56Fe/54Fe (expressed as δ56Fe), and these results are plotted in Figure 2. Changes in the instantaneous Fe isotope fractionation factor between Fe(II)aq and goethite are reflected in changes in the trajectory of δ56Fe values and percent isotopic exchange, and this was evaluated for three different initial δ56Fe values for Fe(II)aq, where contrasts in initial δ56Fe values would highlight different inflections in the exchange trajectories. Our results provide a demonstration that the magnitude of initial kinetic isotope effects, as reflected in inflections in the δ56Fe-% exchange trends (Figure 2), is a function of the extent of sorption of Fe(II)aq, as modified by different pH conditions.
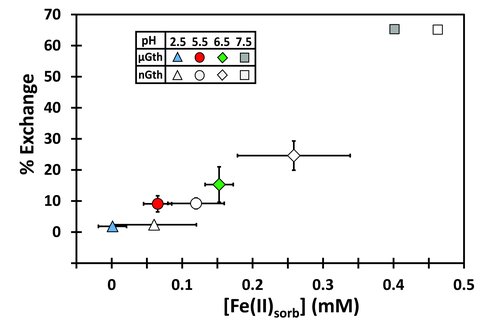
The isotopic exchange rates are dependent upon the extent of Fe(II)sorb (Figure 3), providing strong confirmation that electron transfer is a driving force for isotopic exchange. The smaller the Fe(II) quantity in direct contact with the goethite surface, the smaller number of electron transfer reactions per unit time, the slower the system will come to isotopic equilibrium. Related to these effects is the issue of kinetic isotope fractionation (Figure 2), which we interpret to be generated by non-equilibrium sorption effects early in the experiment that reflect mass-transport differences among the stable Fe isotopes in the boundary layer between the goethite surface and ambient Fe(II)aq.
Our results suggest that although initial kinetic isotope effects occur during Fe(II)aq-goethite interactions, the system does move toward equilibrium. The relatively sluggish Fe isotope exchange kinetics at low-pH suggests that once a Fe isotope signal is “locked in” for Fe oxides, it will be resistant to change under acidic conditions. This in turn indicates that stable Fe isotope signals recoded in oxides formed at low-pH are likely to be retained in the rock record.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Clark Johnson
Project Investigator
Brian Beard
Co-Investigator
Andrew Frierdich
Collaborator
Thiruchelvi Reddy
Collaborator
-
RELATED OBJECTIVES:
Objective 2.1
Mars exploration.
Objective 5.3
Biochemical adaptation to extreme environments
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems