2014 Annual Science Report
 University of Wisconsin
Reporting | SEP 2013 – DEC 2014
University of Wisconsin
Reporting | SEP 2013 – DEC 2014
Project 2F: Silicon Isotopes as a Tracer of the Coupled Fe-Si Cycle in the Archean
Project Summary
Before the appearance of marine Si secreting organisms in the early Phanerozoic, the Precambrian ocean was characterized by high Si concentrations, as demonstrated by unusually abundant Precambrian age chert deposits. The Precambrian oceanic Si cycle was controlled by Si input from continental and hydrothermal sources, Si sorption and precipitation during Fe cycling, and export of Si in chemical precipitates. Reconstruction of the Precambrian Si cycle provides a better understanding of key processes that have governed the Si-rich ocean and associated environment where the earliest life on the Earth originated. Si isotopes are potential tracers for Si cycle where δ30Si values range over 4 ‰ in Precambrian rocks. However, unambiguous interpretation of Precambrian Si isotope data is limited by a lack of knowledge on Si isotope fractionation factors determined for systems applicable to the Precambrian. We have established protocols for high-precision Si isotope analysis, and conducted a series of laboratory experiments to determine Si isotope fractionation factors in simulated systems directly relevant to deposition and preservation of Archean cherts. These experiments represent the first attempt for a mechanistic understanding of Precambrian Si isotope data, and our results highlight the importance of deposition and preservation processes in affecting Si isotopic compositions preserved in Archean sedimentary records.
Project Progress
Precambrian Si isotope compositions were largely controlled by Si chemical precipitation, input of Si with different isotope compositions (e.g., continental versus hydrothermal sources), and later alteration and diagenetic processes associated with silicification. In Precambrian banded iron formations (BIFs) and chert deposits there is a 4‰ spread of Si isotopes (δ30Si), which stands in marked contrast to the narrow range (<0.5) measured in igneous rocks, highlighting the potential of using Si isotopes to reconstruct those processes that controlled the Precambrian marine Si cycle. However, Si isotope fractionation factors are poorly constrained. This prevents a faithful interpretation of Si isotope data in Precambrian records. In this work, we preformed a series of laboratory experiments that determine Si isotope fractionation factors during simulated processes directly relevant to Archean conditions. With the support of the experimentally determined fractionation factors we will be able to provide an interpretative framework for our future Si isotope studies of Archean age.
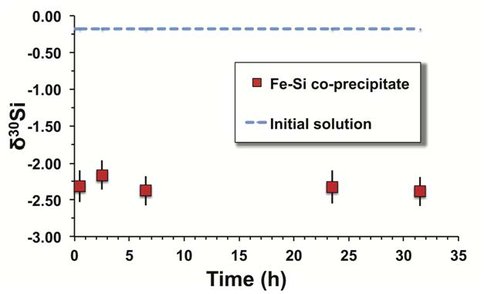
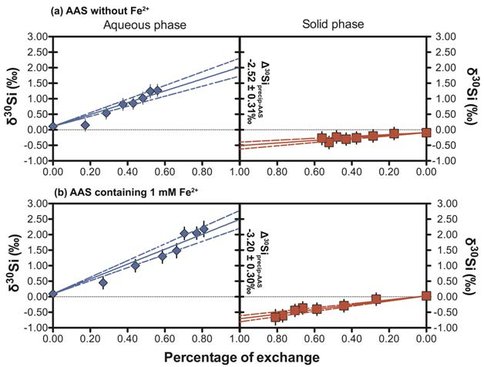
Experiments focused on Si isotope fractionation during co-precipitation of Fe-Si gels, and between amorphous Fe-Si gels and aqueous Si. All experiments were conducted in an artificially prepared medium that mimics Archean seawater (e.g. Si > 20 ppm, seawater-like matrix), rather than in a simple Fe-Si solution. Use of an Archean seawater analog is important because previous studies have revealed distinct Fe isotope fractionation behaviors in such a medium as compared to simples solutions. For Fe-Si co-precipitation experiments, our results show that δ30Si values of amorphous Fe-Si co-precipitates, produced during oxidation of Fe2+ in the AAS at room temperature, are ~2‰ lower than the initial AAS (Δ30Siprecip-AAS = -2.13 ± 0.18‰ (2σ)) (Fig. 1). The Si exchange experiments adopted the so-called three-isotope method, in which aqueous Si (AAS) was spiked with a 29Si-enriched tracer, and then allowed to equilibrate with isotopically normal amorphous Fe-Si gels. This is the first application of the three-isotope method to the Si isotope system that allows for rigorous evaluation of Si isotope exchange and determination of Si isotope fractionation factors at 100% isotope exchange. Two parallel sets of reactors were utilized. The first set used an Fe2+-free 29Si-spiked AAS, and second set used the 29Si-spiked AAS containing ~1 mM aqueous Fe2+. Both solutions were reacted with the same Fe-Si gel at similar pH and ambient conditions in an anaerobic chamber. After ~30 days, the experiment using the Fe-free AAS had 50% Si isotope exchange between the solid and solution. In contrast, the experiment using the Fe-containing AAS had isotopically exchanged~80% in the same amount of time (Fig. 2). Extrapolation to 100% isotope exchange leads to an equilibrium fractionation factor (Δ30Siprecip-AAS) of -2.52 ± 0.31‰ for the experiments without Fe2+ in the AAS, and a larger fractionation factor (Δ30Siprecip-AAS) of -3.20 ± 0.30‰ for the experiments using 1 mM Fe2+ in the AAS (Fig. 2). Several important conclusions can be drawn from these results: (1) Si isotopes in Fe-Si co-precipitates, the assumed precursors of Archean cherts, are easily exchangeable with ambient aqueous Si solution; (2) the presence of aqueous Fe2+ in the solution promotes Si isotope exchange; (3) the presence of Fe2+ in the solution leads to larger Si isotope fractionation during isotope exchange between the solid and solution. This last conclusion may explain the observation that BIF-associated Precambrian cherts have Si isotope compositions more negative than those recorded in coeval pure cherts (Chakrabarti et al., 2012; Marin-Carbonne et al., 2014). Our results highlight the importance to take into account formation and diagenetic processes, which are largely ignored in the published literature, when interpreting Archean Si isotope data.
References Cited:
(1) Chakrabarti et al., 2012. Si isotope variability in Proterozoic cherts. Geochimica et Cosmochimica Acta (91), 187-201.
(2) Marin-Carbonne et al., 2014. The silicon and oxygen isotope compositions of Precambrian
cherts: a record of oceanic paleo-temperatures? Precambrian Research (247), 223-234.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Brian Beard
Project Investigator
Clark Johnson
Co-Investigator
Eric Roden
Co-Investigator
Thiruchelvi Reddy
Collaborator
Xinyuan Zheng
Collaborator
-
RELATED OBJECTIVES:
Objective 4.1
Earth's early biosphere.
Objective 7.1
Biosignatures to be sought in Solar System materials