2014 Annual Science Report
 University of Wisconsin
Reporting | SEP 2013 – DEC 2014
University of Wisconsin
Reporting | SEP 2013 – DEC 2014
Project 2E: Carbonate-Associated Sulfate (CAS) as a Tracer of Ancient Microbial Ecosystems
Project Summary
The chemical compound sulfate is present in ocean water and ratios of its stable isotopes of sulfur and oxygen have varied over geological time and are indicators of global geochemical processes. Other researchers have extracted trace amounts of sulfate from carbonate minerals of various ages as a glimpse into the Earth’s geological past. We are, however, applying this approach to carbonate minerals formed by microbial processes during burial of sedimentary rocks, which we hoped would give information on the microbial ecosystems. We needed to modify and develop existing methods for extracting the trace amounts of sulfate because our samples would be mineralogically much more complex. Initially, just to test the method we tried it on material from the local Monterey Formation rocks, which are of Miocene age (approx. 13 My old) and were delighted to find that the results enabled us to see the workings of a very complex microbial ecosystem with at least three different sorts of metabolism operating.
Project Progress
At the end of last year’s work we had adapted previously published methods and developed a protocol for extraction of trace sulfate from carbonates suitable for our particular application, which involves carbonate minerals much more chemically and mineralogically diverse than those used to date (Theiling and Coleman, 2015a). To test the method we had collected samples from a coastal section of the relatively local Monterey formation near Santa Barbara, CA. Not only did the new extraction technique work, but the results also gave a very exciting, detailed insight into the microbial ecosystem operating during early burial of these sediments.
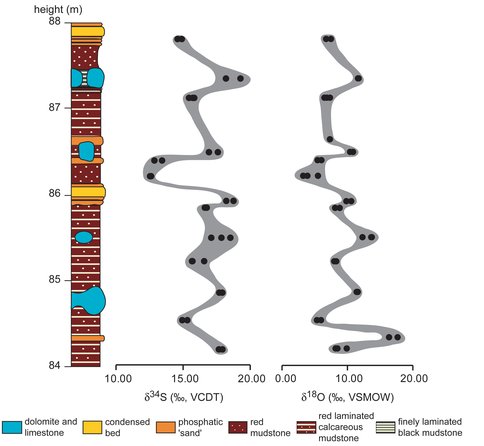
We analyzed the sulfur and oxygen isotope compositions of the trace sulfate extracted from nodular and bedded carbonates and from phosphate rocks (Theiling and Coleman, 2015b).
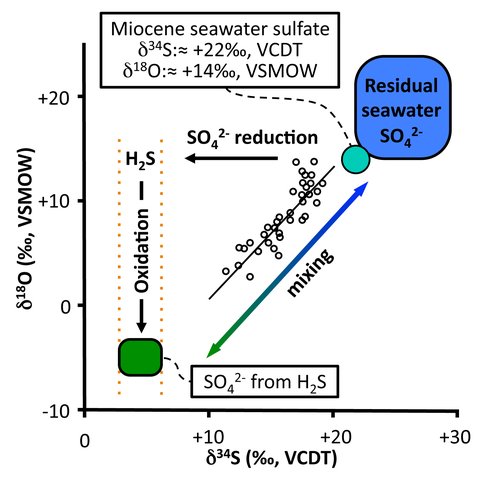
Two observations can be made immediately from the data shown in Fig 1, where the stratigraphic section was adapted from that of Föllimi et al., 2005). There is a wide variation in isotope values and there seems to be a correlation between the sulfur and oxygen isotope results. Given that Miocene age seawater had sulfur and oxygen isotope compositions of +22‰ and +14‰, respectively, it is clear that the sulfate is not pristine seawater but is the result of microbial, diagenetic processes operating during sediment burial. Examination of the correlation trend initially seems to imply a mixture between seawater sulfate and an end member with much more negative sulfur oxygen isotope compositions.
While sulfate reduction preferentially removes the lighter isotopes of sulfur and oxygen, yielding isotopically more positive values in residual sulfate, these values which are more negative than the original seawater arise from a completely different process. Microbial sulfate reduction produces sulfide with a much more negative composition than the original sulfate and can be precipitated with iron as pyrite, which is found in this formation. However, if there is insufficient iron, excess dissolved sulfide will diffuse upwards where aerobic microbes may oxidize it with oxygen, producing sulfate with negative sulfur and oxygen isotope compositions. In fact, the extrapolated end member composition would coincide with the sulfur isotope values for pyrite in this formation, reported by Lloyd et al. (2012). We have plotted the sulfate produced by oxidation of sulfide with the oxygen isotope value we determined experimentally in lab experiments on microbial oxidation of pyrite with atmospheric oxygen. This hypothesis is supported by the presence of phosphate in the section, which is favored over carbonate precipitation at lower pH (Nathan and Sass, 1981). In this environment sulfide oxidation would lower pH slightly.
We undertook field work to collect both Early Jurassic age and very recently formed iron carbonate concretions.
For the latter, still forming in 75 year old sediments, it is possible to make environmental analyses of the water composition and microbial ecosystem as well as the geochemistry and mineralogy. We have full mineralogical and chemical analyses, environmental DNA and phospholipid fatty acid (PLFA) data and have just started the CAS extractions so that we can integrate all the observations.
References
Föllimi, K.B., Badertscher, C., de Kaenel, E., John, C.M., Adatte, T., and Steinmann, P. (2005) Phosphogenesis and organic-carbon preservation in the Miocene Monterey Formation at Naples Beach, California—The Monterey hypothesis revisited. Geological Society of America Bulletin, 117, 589-619.
Loyd, S.J., Berelson, W.M., Lyons, T.W., Hammond, D.E., Corsetti, F.A., (2012) Constraining pathways of microbial mediation for carbonate concretions of the Miocene Monterey Formation using carbonate-associated sulfate. Geochimica et Cosmochimica Acta, 78, 77-98.
Nathan, Y. & Sass, E. (1981) Stability relations of apatites and calcium carbonates. Chem. Geol. 34, 103-111.
Theiling, B.P. and Coleman, M. (2015) Refining the extraction methodology of carbonate associated sulfate: Evidence from synthetic and natural carbonate samples. Chem Geol, (in review).
Theiling, B.P. and Coleman, M. (2015) Microbial consumption of mid-Miocene “missing” buried organic carbon. Nat Geosci.(in prep).
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Max Coleman
Project Investigator
Bethany Theiling
Co-Investigator
James Boles
Collaborator
Amanda aka Meg Galsworthy
Collaborator
Robert Mortimer
Collaborator
-
RELATED OBJECTIVES:
Objective 5.2
Co-evolution of microbial communities
Objective 6.1
Effects of environmental changes on microbial ecosystems
Objective 7.1
Biosignatures to be sought in Solar System materials