2014 Annual Science Report
 University of Wisconsin
Reporting | SEP 2013 – DEC 2014
University of Wisconsin
Reporting | SEP 2013 – DEC 2014
Project 2D: Magnesium Isotopes in Carbonates as a Tracer of Marine Conditions in the Early Earth
Project Summary
Massive dolomitization events affect seawater Mg concentration and have a profound influence on the carbonate cycle in seawater that ultimately controls seawater chemistry and atmospheric carbon dioxide levels. The Mg isotope fractionation factor between dolomite and aqueous Mg has been experimentally constrained at 130, 160, and 220 degrees C to derive a temperature-dependent fractionation factor. This Mg isotope fractionation function has been determined to allow evaluation of the Mg isotope composition of fluids that have produced dolomite. Based on these new data it is now possible to infer secular changes in seawater Mg isotope compositions based on the analysis of sedimentary dolomites. This information can be used to infer changes in the intensity of dolomitization which removes Mg from seawater and tectonism which controls mid ocean ridge hydrothermal circulation that largely removes Mg from seawater as well as impacts weathering.
Project Progress
Dolomite [MgCa(CO3)2] is an important Mg-bearing carbonate mineral, and dolomite-bearing lithologies comprise a major component of the ancient carbonate record. The relative abundance of dolomite in sedimentary rocks has varied throughout geological time, comprising the major proportion of carbonate rocks deposited in the Ordovician to Early Carboniferous and Triassic to Mid Cretaceous, but lesser proportions at other times. Cyclical change in dolomitization intensity has been broadly correlated with long-term rhythmic changes in sea level (e.g., Vail et al., 1977), mineralogy of primary seawater calcium carbonates (e.g., Sandberg, 1983; Stanley and Hardie, 1998), seawater chemistry (e.g., Lowenstein et al., 2001), and atmospheric pCO2 levels (e.g., Fischer, 1984), suggesting that sedimentary dolomite may contain clues to understanding the secular changes in Earth’s climatic and environmental parameters (Burns et al., 2000).
Stable Mg isotopes are an emerging geochemical tool, and it has great potential for studying dolomite-related problems. For example, controversy remains on the cause of secular changes in seawater Mg/Ca ratio in the Phanerozoic, where some workers propose that it was caused by variations in the intensity of dolomite formation (Holland, 2005; Wilkinson and Algeo, 1989), but others argue that it was mainly controlled by the intensity of mid-ocean ridge hydrothermal activity (Hardie, 1996; Spencer and Hardie, 1990). Understanding the controls of Mg isotope fractionation between aqueous Mg and carbonate minerals is a critical first step in allowing one to use Mg isotopes to infer controls on the Mg/Ca ratio in seawater. We have conducted hydrothermal experiments to study the Mg isotope exchange and fractionation factor between aqueous fluids and dolomite at 130, 160, and 220 oC to derive a temperature-dependent fractionation factor of Δ26Mgdolo-aq = -0.1554(±0.0096) ×106/T2, where T is in Kelvin.
The dolomite aqueous Mg fractionation factor (Δ26Mgdolo-aq) was determined using a combination of isotope exchange and synthesis experiments. This approach was done to allow us to better asses kinetic versus equilibrium fractionation effects. The isotope exchange experiments used a three isotope method to track the Mg isotope exchange rate with time allowing us to extrapolate to the Mg isotope fractionation factor at 100% isotope exchange. The dolomite synthesis experiments used aragonite, calcite or nesquehonite (hydrated Mg carbonate) starting minerals and solutions containing aqueous Mg and Ca under hydrothermal conditions at 220, 160, and 130 °C.
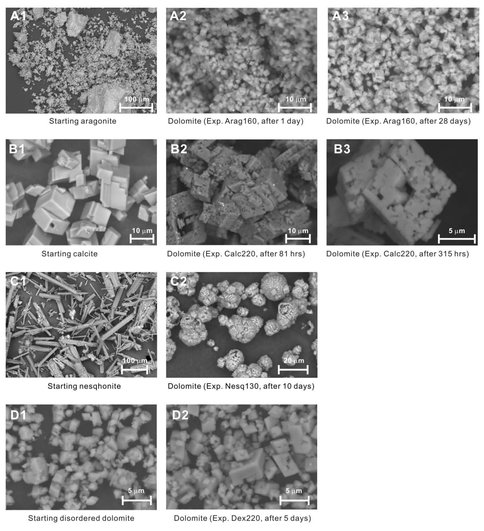
There are distinct differences in morphology of the synthesized dolomite between experiments with different starting materials. Synthesis experiments using aragonite as the starting material typically produced homogenous, fine-grained (1-5 μm in size), well dispersed, euhedral rhombohedrons of dolomite (Figure 1, image A2, A3), despite the huge variation in grain size of starting aragonite (a few to over 100 μm in size, Figure 1, image A1). Synthesis experiments using calcite as the starting material, however, produced dolomite aggregates that apparently retained the 5-20 μm -sized rhomboheral morphology of original calcite crystals, but with numerous sub-micron sized cavities/pits (Figure 1, image B2, B3). Such morphology has been reported in
previous dolomite synthesis experiments using calcite, and was referred to as the “Swiss cheese” texture (Katz and Matthews, 1977). Synthesis experiments using nesquehonite as the starting material produced spherical aggregates of fine-grained crystals of dolomite (and Mg-calcite for 130°C experiment), and the diameters of spheres ranged from 5 to 30 μm (Figure 1, image C2), fundamentally different from the rod-like shape of the starting nesquehonite.
These differences in morphology reflect differences in how the dolomite formed. For example for the experiments that used calcite as the starting material, the dolomite crystal aggregates inherited the original calcite morphology, implying the dolomite formed through a mineral replacement process. Disordered dolomite, Mg-bearing calcite, and calcite share a common lattice configuration (space group: R-3c) despite differences in unit-cell volumes (Zhang et al., 2010), which facilitates cation replacement/exchange (Mg2+ for Ca2+) for dolomitization of calcite. Addtionally, because the unit-cell volume of calcite (367.8 Å3) is larger than that of dolomite (319.4 Å3), the mineral shrinks during replacement reaction, producing cavities as shown in SEM images (Figure 1, images B2 and B3). The cavities, in turn, serve as fluid channels that allow reaction front to advance to inner portions of calcite grains.
In contrast, for experiments that used aragonite or nesquehonite as the starting material, the dolomite did not retain the original carbonate morphology, but occurred as homogenous fine-grained crystals or their aggregates. These observations suggest that the dolomite crystals probably formed via a homogeneous precipitation mechanism. Aragonite has a different lattice configuration (space group: Pcmn) than that of dolomite, therefore aragonite does not serve as a template for dolomite growth. More importantly, because the unit-cell volume of aragonite (226.2 Å3) is significantly smaller than that of dolomite (319.4 Å3), the newly formed dolomite has to detach from its aragonite precursor during reaction due to lattice expansion. This explains the homogenous and fine-grained nature of dolomite produced from aragonite in the synthesis experiments (Figure 1, images A2 and A3), where large (up to 100 μm) aragonite grains were converted to small dolomite grains . Nesquehonite has a unit-cell volume (501.2 Å3) that is more than 50% larger than that of the dolomite. This volume difference likely explains the reason why the dolomite does not retain the original crystal shape of nesquehonite (Figure 1, image C2) because replaced crystals would collapse with such high void proportion.
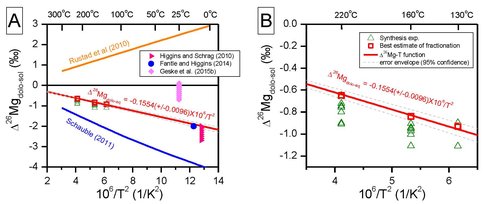
In these synthesis reactions dolomite formed via different process and it is noteworthy that that the measured fractionation factors for the longest duration synthesis reactions agree with the measured fractionation factor for the isotope exchange experiments done at 220 °C and 160 °C. The best estimates of equilibrium fractionation factors therefore can be calculated by combining those results from exchange experiments and synthesis experiments and the weighted average Δ26Mgdolo-aq fractionation at 220 °C is 0.65±0.04 ‰ (95% confidence, n=3, MSWD=1.18), and the weighted average Δ26Mgdolo-aq fractionation at 160 °C is -0.84±0.04 ‰ (95% confidence, n=2, MSWD=1.05). The fractionations from synthesis experiments and exchange experiments at 130°C is poorer relative to those at higher temperatures, however, the solid phases in products from synthesis experiments at 130 °C were not pure dolomite; therefore, only the Δ26Mgdolo-aq fractionation (-0.93±0.05 ‰, 2SD, or 95% confidence) of the longest duration exchange experiment was used (88.8% Mg isotope exchange, and pure dolomite in solid phase). The fact that synthesis experiments which produced dolomite from using two different pathways (e.g., mineral replacement for calcite starting experiments or homogenous precipitation for aragonite and nesquehonite starting experiments) and the exchange experiments yield concordant results is taken as strong evidence that these measured fractionations represent equilibrium isotope exchange. Extrapolation of these results on an Arrenhius plot (Figure 2) yields Δ26Mgdolo-aq = 0.1554(±0.0048) ×106/T2, where T is in Kelvin.
Equilibrium fractionation factors of Mg isotopes between dolomite and aqueous solution have been calculated in two independent studies by Rustad et al. (2010) and Schauble (2011). Rustad et al. (2010) predicted positive Δ26Mgdolo-aq fractionations, whereas Schauble (2011) predicted negative Δ26Mgdolo-aq fractionations (Figure 2). Experimentally calibrated fractionation factors for dolomite from this study lie between the two predictions, but are most consistent with Schauble (2011) (Figure 2). The origin of these differences in unknown but it does highlight the importance that experimentally measured fractionation factors are important pieces of ground truth data to allow one to evaluate calculated fractionation factors. Our experimentally measured fractionation factors agree well with empirically derived fractionation factors between dolomite and aqueous Mg from ODP drill cores (Figure 2). Higgins and Schrag (2010) analyzed Mg isotope compositions of pore water and dolomite from three ODP drill cores, and based on reactive transport modeling, Higgins and Schrag (2010) concluded that a Δ26Mgdolo-aq fractionation factor of -2.0 to -2.7 ‰ is needed to explain the vertical profile of δ26Mg values of pore water and dolomite. Similar conclusions have been drawn in a more recent study on another ODP site, where a Δ26Mgdolo-aq fractionation factor of -2.0 ‰ is deduced from modeling of the observed Δ26Mg values along the drill core (Fantle and Higgins, 2014). .
REFERENCES CITED:
Burns, S.J., McKenzie, J.A., Vasconcelos, C., 2000. Dolomite formation and biogeochemical cycles in the Phanerozoic. Sedimentology 47, 49-61.
Fantle, M.S., Higgins, J., 2014. The effects of diagenesis and dolomitization on Ca and Mg isotopes in marine platform carbonates: Implications for the geochemical cycles of Ca and Mg. Geochimica et Cosmochimica Acta 142, 458-481.
Fischer, A.G., 1984. The two Phanerozoic supercycles, in: Berggren, W.A., Vancouvering, J.A. (Eds.), Catastrophies in Earth History. Princeton University Press, Princeton, pp. 129-148.
Hardie, L.A., 1996. Secular variation in seawater chemistry: An explanation for the coupled secular variation in the mineralogies of marine limestones and potash evaporites over the past 600 m.y. Geology 24, 279-283.
Higgins, J.A., Schrag, D.P., 2010. Constraining magnesium cycling in marine sediments using magnesium isotopes. Geochimica et Cosmochimica Acta 74, 5039-5053.
Holland, H.D., 2005. Sea level, sediments and the composition of seawater. Am J Sci 305, 220-239.
Katz, A., Matthews, A., 1977. The dolomitization of CaCO3: an experimental study at 252–295°C. Geochimica et Cosmochimica Acta 41, 297-308.
Lowenstein, T.K., Timofeeff, M.N., Brennan, S.T., Hardie, L.A., Demicco, R.V., 2001. Oscillations in Phanerozoic Seawater Chemistry: Evidence from Fluid Inclusions. Science 294, 1086-1088.
Rustad, J.R., Casey, W.H., Yin, Q.-Z., Bylaska, E.J., Felmy, A.R., Bogatko, S.A., Jackson, V.E., Dixon, D.A., 2010. Isotopic fractionation of Mg2+(aq), Ca2+(aq), and Fe2+(aq) with carbonate minerals. Geochimica et Cosmochimica Acta 74, 6301-6323.
Sandberg, P.A., 1983. An oscillating trend in Phanerozoic non-skeletal carbonate mineralogy. Nature 305, 19-22.
Schauble, E.A., 2011. First-principles estimates of equilibrium magnesium isotope fractionation in silicate, oxide, carbonate and hexaaquamagnesium(2+) crystals. Geochimica et Cosmochimica Acta 75, 844-869.
Spencer, R.J., Hardie, L.A., 1990. Control of seawater composition by mixing of river waters and mid-ocean ridge hydrothermal brines. Spec. Publ. – Geochem. Soc. 19, 409-419.
Stanley, S.M., Hardie, L.A., 1998. Secular oscillations in the carbonate mineralogy of reef-building and sediment-producing organisms driven by tectonically forced shifts in seawater chemistry. Palaeogeography, Palaeoclimatology, Palaeoecology 144, 3-19.
Vail, P.R., Mitchum, R.M., Thompson, S., 1977. Seismic stratigraphy and global changes of sea-level, Part 4: Global cycles of relative changes of sea level. American Association of Petroleum Geologist Memoir 26, 83-97.
Wilkinson, B.H., Algeo, T.J., 1989. Sedimentary carbonate record of calcium-magnesium cycling. American Journal of Science 289, 1158-1194.
Zhang, F., Xu, H., Konishi, H., Roden, E.E., 2010. A relationship between d104 value and composition in the calcite-disordered dolomite solid-solution series. American Mineralogist 95, 1650-1656.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Brian Beard
Project Investigator
Clark Johnson
Co-Investigator
Weiqiang Li
Collaborator
Huifang Xu
Collaborator
-
RELATED OBJECTIVES:
Objective 4.1
Earth's early biosphere.
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems