2014 Annual Science Report
 University of Wisconsin
Reporting | SEP 2013 – DEC 2014
University of Wisconsin
Reporting | SEP 2013 – DEC 2014
Project 2C: Developing the 13C-18O Clumped Isotope Thermometer
Project Summary
One of the critical parameters in understanding the evolution of the Earth is the temperature of the oceans. Proposals, for example, that early life was hyperthermophilic would be supported if there was evidence for a “hot early ocean”, which some stable isotope data support. However, arguments against a “hot early ocean” include lower solar luminosity early in Earth history, and evidence for widespread glaciations. A new geothermometer is based on the enhanced thermodynamic stability of “clumped isotopes” (e.g., 13C-18O bonds) in carbonate minerals, which exhibits a temperature dependence. However, attempts to calibrate this temperature dependence have identified kinetic isotope effects that can give apparently anomalous results. In this project, experiments were designed to systematically probe formation rate effects on 13C-18O bonding during calcite mineral lattice assembly from aqueous solutions. Preliminary results do not show a correlation between precipitation rate and 13C-18O bonding over the range investigated but do provide evidence that is being used to deconvolute and identify physiochemical conditions and processes that lead to disequilibrium in 13C-18O bonding during carbonate mineral formation.
Project Progress
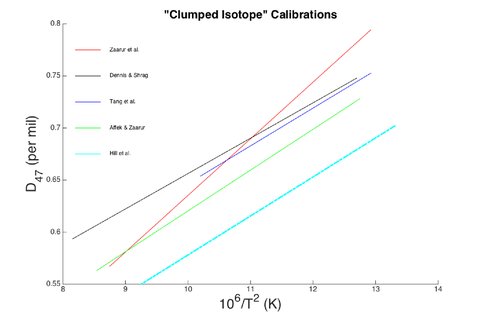
The study of “clumped isotopes” involves quantification of the preferential bonding between rare stable isotopes (e.g., 13C bound to 18O) in multiply substituted isotopologues as compared to stochastic (random) bonding predicted for that chemical compound or mineral lattice. Because clumped isotopes are defined by two or more isotopes of an element of relatively high mass bonded together, thermodynamic stability is further increased compared to singly substituted isotopologues. This stability is inherent to a material and is independent of the bulk isotope composition of the mineral, as well as the composition of a fluid from which the mineral formed. This later component sets clumped isotope thermometry apart from the traditional stable isotope approaches, where the fluid composition must be estimated to obtain a temperature. Essentially, clumped isotopes are an “internal thermometer”. Recent work intended to develop this thermodynamic property as a temperature record has revealed kinetic and vital effects that can produce inaccurate temperature signals. A number of experimental and field-based studies as well as theoretical calculations have been performed in an attempt to reliably calibrate the temperature dependence of 13C-18O bonding during mineral formation. Figure 1 illustrates the wide variability of such calibrations.
Project 2C has been designed to explore theories put forth to explain calibration discrepancies. Specifically, kinetic isotope effects are suspected to cause disequilibrium and prevent accurate temperature records and full 13C-18O bonding. These experiments were designed to systematically probe formation rate effects on 13C-18O bonding during calcite mineral lattice assembly from aqueous solutions. Calcite has been synthetically precipitated using a chemostat technique whereby solution conditions such as ion concentrations, pH, CO2 partial pressure, and saturation index are held constant during mineral formation. This method is designed to maintain a constant growth rate and level of supersaturation as well as provide a precipitation mechanism that does not rely on homogenous nucleation, CO2 diffusion, or CO2 degassing. These conditions are necessary in order to maintain the constant precipitation rate desired during a given experiment.
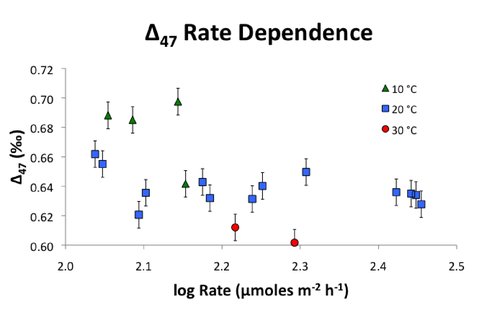
Forty-six synthetic calcite precipitations were performed in 2014. Most calcite formed at 20°C. Reactant concentrations ranged from 6 mM to 10 mM for both Ca2+ and HCO3-. Solution pH during precipitation ranged from 6.5 to 7 resulting in saturation indices between 1 and 2. Experiments lasted from three to eight days resulting in precipitation log rates ranging from approximately 2 to 2.5 μmol/m2/hr. Figure 2 is a plot of 13C-18O bonding (denoted Δ47, which is the deviation of 13C18O16O species relative to random distribution) as a function of formation rate and shows values for experiments that have been analyzed multiple times. Δ47 values determined for 20°C experiments averaged 0.639 ± 0.014‰ and do not correlate with precipitation rate over the range investigated. Oxygen isotope fractionation factors (αcalcite-water) were found to be slightly higher than those found previously, suggesting precipitation conditions approaching equilibrium. The preliminary results provide evidence that can be used to deconvolute and identify physiochemical conditions and processes that lead to disequilibrium in 13C-18O bonding during carbonate mineral formation.
Most experiments conducted at 10°C and 30°C have only been analyzed once for Δ47. The 13C-18O bonding during these experiments follow the general trend of decreasing frequency with increasing formation temperature. Replicate analyses are. Additional work planned for this project includes the execution of further experiments and analyses that will expand the range of temperatures and/or precipitation rates explored.
Publications
-
Levitt, N. P. (2014). Sample matrix effects on measured carbon and oxygen isotope ratios during continuous-flow isotope-ratio mass spectrometry. Rapid Commun. Mass Spectrom., 28(21), 2259–2274. doi:10.1002/rcm.7019
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Clark Johnson
Project Investigator
Brian Beard
Co-Investigator
John Eiler
Co-Investigator
Nicholas Levitt
Collaborator
-
RELATED OBJECTIVES:
Objective 4.1
Earth's early biosphere.
Objective 6.1
Effects of environmental changes on microbial ecosystems
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems