2014 Annual Science Report
 University of Wisconsin
Reporting | SEP 2013 – DEC 2014
University of Wisconsin
Reporting | SEP 2013 – DEC 2014
Project 2B: Modeling the Role of Adsorbed Hydrogen Sulfide in Weakening Mg-Water Complex on Dolomite (104) Surface
Project Summary
Hydrogen sulfide is a major product of sulfate-reducing bacteria (SRB) in marine environment. To characterize the role of dissolved hydrogen sulfide and SRB in promoting dolomite crystallization, ab initio simulations based on density functional theory (DFT) were carried out to study the thermodynamics of competitive adsorption of hydrogen sulfide and water on dolomite (104) surfaces from solution. The results indicate that hydrogen sulfide adsorbed on the (104) surface increases the Mg2+-H2O distances on surrounding surface sites and thus weakens the electrostatic interactions between surface Mg[2^+^] and water molecules. The adsorbed hydrogen sulfide will promote surface water removal and anhydrous Ca-Mg-carbonate and dolomite crystallization at low temperature.
Project Progress
The key kinetic barrier to dolomite formation is related to the surface Mg2+-H2O complex, which hinders binding of surface Mg2+ ions to the CO32- ions in solution. It has been proposed that this reaction can be catalyzed by dissolved hydrogen sulfide. We find that water is thermodynamically more stable on the surface with the difference in adsorption energy of -13.6 kJ/mol (in vacuum) and -12.8 kJ/mol (in aqueous solution).
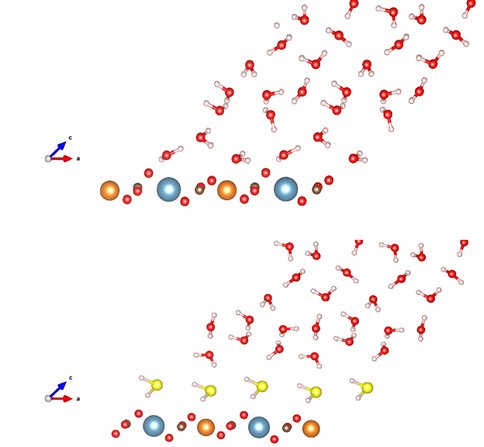
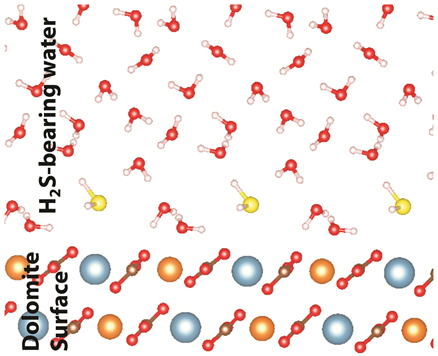
Although water is more energetically stable on dolomite surface than aqueous hydrogen sulfide, our two-phase adsorption model predicts a small amount of H2S adsorbed on the surface depending on the adsorption energy difference and pH of solution (affects the concentration of aqueous H2S). At pH of 7.0~8.2 where the modern dolomite is precipitating, 6%~50% of dissolved sulfide are in aqueous H2S phase. Usually more than 5mM dissolved sulfide was measured in pore water from some modern dolomite site. In order to test the effect of the surface H2S on the Mg2+-water complex, a mixed layer of 50% H2S and 50% H2O molecules was placed above the dolomite surface in the presence of bulk water. We tested multiple configurations by using the same method as described in previous section and the lowest energy structure was obtained by inserting an extra water molecule between the bulk water and the adsorbed first layer. In the adsorbed mixed layer, the Mg2+-Ow distance increases from ~2.18 Å to ~2.23 Å compared to the respective case of pure layers (Table 1). Therefore, the adsorbed hydrogen sulfide will enhance surface water removal and dolomite crystallization. The surface water and adsorbed hydrogen sulfide on dolomite (104) surface, and a surface with mixed water and hydrogen sulfide are illustrated in figures below. Similar works on the effect of adsorbed polysaccharides on surface water removal is in progress.
Table 1. Energies and distances between surface Mg and O of adsorbed water for adsorption of a) 1 mono-layer (ML) of H2O in solution; b) 1 ML of H2S in solution; and c) a mixed H2O and H2S layer in solution.
Publications
-
Shen, Z., Konishi, H., Szlufarska, I., Brown, P. E., & Xu, H. (2014). Z-contrast imaging and ab initio study on “d” superstructure in sedimentary dolomite. American Mineralogist, 99(7), 1413–1419. doi:10.2138/am.2014.4647
-
Shen, Z., Liu, Y., Brown, P. E., Szlufarska, I., & Xu, H. (2014). Modeling the Effect of Dissolved Hydrogen Sulfide on Mg 2+ –Water Complex on Dolomite {104} Surfaces. The Journal of Physical Chemistry C, 118(29), 15716–15722. doi:10.1021/jp5028417
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Huifang Xu
Project Investigator
Philip Brown
Collaborator
Zhizhang Shen
Collaborator
Izabela Szlufarska
Collaborator
-
RELATED OBJECTIVES: