2014 Annual Science Report
 University of Wisconsin
Reporting | SEP 2013 – DEC 2014
University of Wisconsin
Reporting | SEP 2013 – DEC 2014
Project 1D: Iron Biogeochemistry in Chocolate Pots Hot Spring, Yellowstone National Park
Project Summary
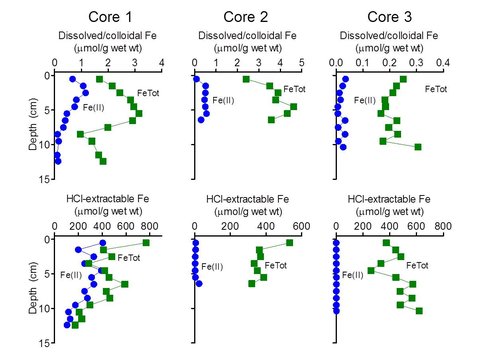
Small cores were collected from six locations along a transect following the main fluid flow path at Chocolate Pots (CP) hot spring, Yellowstone National Park. The cores were sectioned at 1 cm intervals, and the solids subjected to sequential extraction to isolate different Fe pools. The results showed that cores proximal to the vent outlet contained significant quantities of dissolved/colloidal and HCl-extractable reduced (ferrous) iron [Fe(II)]. Fe recovered from the other cores was present entirely as Fe(III). The most likely explanation for these observations is that internal generation of Fe(II) via microbial reduction is taking place in deposits proximal to the vent. This interpretation is consistent with rapid Fe(II) production during anaerobic incubation of near-vent deposits. Our results provide direct evidence of Fe(III) oxide reduction in deposits proximal to the main vent at CP, and to our knowledge represent the first demonstration of in situ Fe(III) reduction in a circumneutral-pH geothermal environment analogous to those which may have been present on the ancient Earth and Mars. Preliminary stable Fe isotope measurements on the dissolved/colloidal and 0.5M HCl-extractable Fe fractions in the CP cores suggests that Fe(III) reduction influences the isotopic composition of Fe phases proximal to the vent. A comprehensive analysis of all Fe phases in the cores is underway and will be used to develop conceptual models of controls on the stable Fe isotope composition preserved in the hot spring deposits.
Project Progress
Iron (Fe) biogeochemical cycling in circumneutral pH systems is an increasingly important astrobiological target in light of recent discoveries on Mars by Curiosity (Grotzinger et al., 2014). Previous research into microbial ferric iron [Fe(III)] reduction in Yellowstone National Park geothermal springs has focused on high temperature, low pH environments where microbial communities are able to utilize soluble Fe(III) as an electron acceptor for respiration (e.g. Kozubal et al., 2012). Much less attention has been paid to Fe(III)-reducing microbial communities in lower temperature, circumneutral pH environments, where solid phase Fe(III) oxides are the dominant forms of ferric iron.
This study represents the first detailed exploration of Fe biogeochemistry in the warm (ca. 40-60 C), circumneutral pH (ca. 6.0-6.5) Chocolate Pots hot springs (CP) in Yellowstone National Park. The work was motivated by the idea that internal Fe redox cycling driven by microbial Fe(III) reduction could influence patterns of Fe isotope fractionation along the fluid flow path, e.g. by mobilizing dissolved and/or colloidal Fe(II) from a relatively heavy pool of deposited Fe(III) oxides. This process could lead to redistribution of stable Fe isotopes compared to what would be expected for a system in which fractionation is associated only with Fe(II) oxidation and Fe(III) oxide precipitation (Wu et al., 2013). Such internal Fe redox cycling has important implications for interpretation of Fe isotope fractionation patterns as a signature of microbial Fe redox metabolism. Previous NAI-funded work has documented the presence of substantial numbers of Fe(III)-reducing organisms in CP materials, recovered Fe(III)-reducing enrichment cultures capable of sustained reduction of native CP Fe(III) oxide-silica coprecipitates, and demonstrated that isotopically light Fe(II) is generated during microbial reduction of the coprecipitates (Fortney et al., 2015)
Small cores were collected from six locations along a transect following the main fluid flow path at CP, extending from the vent outlet to within a few meters of Gibbon River into which the hot spring discharges.
The cores were sectioned at 1 cm intervals, and the solids subjected to sequential extraction to isolate different Fe pools. Working in an anaerobic chamber, a few grams of wet material was suspended in a few mL of anoxic spring fluid. The suspended material was agitated and then centrifuged. The supernatant contained a mixture of dissolved (aqueous) and colloidal constituents that did not pellet-out with the bulk solids. The abundance of colloidal material (very small Fe-Si coprecipitates) was sufficiently high, given the relatively small volume of sample available, so as to make it impractical to filter the supernatant; hence, Fe in this phase represents a mixture of dissolved and colloidal (nm-sized) particulates. The bulk pelleted material was subjected to sequential extraction with 0.01M and 0.5M HCl. The purpose of the 0.01M HCl fraction was to recover primarily Fe(II) phases for Fe stable Fe isotope analysis. Since the Fe isotope analyses are not yet completed, Fe data for the 0.01M HCl and 0.5M HCl extracts were summed for this report.
The results showed that only cores 1 and 2, i.e. those most proximal to the vent outlet, contained significant quantities of dissolved/colloidal and HCl-extractable Fe(II) (Fig. 2). Fe recovered from core 3 (Fig. 2) and the other three cores (data not shown) was present entirely as Fe(III).
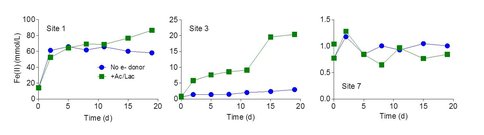
Very high (> 100 μmol HCl-extractable Fe(II) per g wet weight) concentrations of Fe(II) were detected in core 1. Core 2 contained lower (3-25 μmol Fe(II)/g) but still substantial amounts of reduced Fe. There are two possible explanations for the presence of Fe(II) in cores proximal to the vent. First, it could be residual Fe(II) from the vent fluid associated with the solids that precipitate and accumulated in the vicinity of the vent. However, this seems unlikely given that the abundance of Fe(II) in the vent outflow (ca. 0.1 mM) is 10-1000 times lower than bulk Fe(II) abundance in the core solids. A more likely explanation is internal generation of Fe(II) via microbial reduction. To examine this possibility, small quantities of materials from the upper 2 cm at sites 1, 3, and 7 were incubated under anaerobic conditions with or without addition of exogenous organic substrates (a mixture of 2 mM acetate and 2 mM lactate). Concentrations of 0.5M HCl-extractable Fe(II) were followed over a 20 day period.
Rapid Fe(II) production was observed in in vitro Fe(III) reduction experiments conducted with both unamended and acetate/lactate-amended material from core 1, indicating the presence of relatively large quantities of endogenous electron donors (Fig. 3). In contrast, the rate of Fe(II) production in unamended material from core 3, although detectable, was much lower than in the presence of added electron donor. No significant reduction was observed in the materials from core 7, with or without exogenous electron donor, indicating a paucity of Fe(III)-reducing organisms in the core 7 materials. Both of the latter results are consistent with the very low abundance of Fe(II) observed in the corresponding core samples (see Fig. 1). Together these findings indicate that Fe(II) oxidation is likely to be the dominant Fe redox metabolic pathway at locations greater than a few meters from the vent source.
Our results provide direct evidence of Fe(III) oxide reduction in deposits proximal to the main vent at CP, and to our knowledge represent the first demonstration of in situ Fe(III) reduction in a circumneutral-pH geothermal environment analogous to those which may have been present on the ancient Earth and Mars (Cockell et al., 2011). Although the energy source (electron donors) responsible for driving Fe(III) reduction are at present unknown, it seems likely that recycling of chemolithoautotrophic Fe(II)-oxidizing microbial biomass could be involved, as has been inferred for non-geothermal groundwater Fe seep environments (Blöthe and Roden, 2009; Roden et al., 2012). The presence of Fe(III) reduction activity has the potential to generate distinct stable Fe isotopic signatures in modern and ancient sedimentary environments (Johnson et al., 2008). Preliminary stable Fe isotope measurements on the dissolved/colloidal and 0.5M HCl-extractable Fe fractions in the CP cores suggests that Fe(III) reduction does in fact influence the isotopic composition of Fe phases proximal to the vent (cores 1 and 2; data not shown). A comprehensive analysis of all Fe phases in the cores is underway and will be used to develop conceptual models of controls on patterns of stable Fe isotope composition preserved in the hot spring deposits.
References
Blöthe, M., and E. E. Roden. 2009. Microbial iron redox cycling in a circumneutral-pH groundwater seep. Appl. Environ. Microbiol. 75:468-473.
Cockell, C. S., S. L. Cady, and N. McLoughlin. 2011. Volcanism and astrobiology: life on Earth and beyond. Astrobiology 11:583-584.
Fortney, N. W., B. J. Converse, S. He, B. L. Beard, C. M. Johnson, E. S. Boyd, and E. E. Roden. 2015. Microbial Fe(III) oxide reduction potential in Chocolate Pots hot springs, Yellowstone National Park. Manuscript in preparation.
Grotzinger, J. P., D. Y. Sumner, L. C. Kah, K. Stack, S. Gupta, L. Edgar et al. 2014. A habitable fluvio-lacustrine environment at Yellowknife Bay, Gale Crater, Mars. Science 343:DOI: 10.1126/science.1242777.
Johnson, C. M., B. L. Beard, and E. E. Roden. 2008. The iron isotope fingerprints of redox and biogeochemical cycling in the modern and ancient Earth. Ann. Rev. Earth Planet. Sci. 36:457-493.
Kozubal, M. A., W. P. Inskeep, and R. E. Macur. 2012. Geomicrobiology of iron oxide mats from acidic geothermal springs in Yellowstone National Park: Microbial community structure, isolation of novel species and mechanisms of iron oxidation. Front. Microbiol. 3:109.
Roden, E., J. M. McBeth, M. Blothe, E. M. Percak-Dennett, E. J. Fleming, R. R. Holyoke et al. 2012. The microbial ferrous wheel in a neutral pH groundwater seep. Front. Microbiol. 3:172.
Wu, L., R. P. Poulson-Brucker, B. L. Beard, E. E. Roden, and C. M. Johnson. 2013. Iron isotope characteristics of hot springs at Chocolate Pots, Yellowstone National Park. Astrobiology 13:1091-1101.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Eric Boyd
Co-Investigator
Eric Roden
Co-Investigator
Brian Beard
Collaborator
Nathan Fortney
Collaborator
Clark Johnson
Collaborator
-
RELATED OBJECTIVES:
Objective 2.1
Mars exploration.
Objective 4.1
Earth's early biosphere.
Objective 5.3
Biochemical adaptation to extreme environments
Objective 7.1
Biosignatures to be sought in Solar System materials