2014 Annual Science Report
 University of Southern California
Reporting | SEP 2013 – DEC 2014
University of Southern California
Reporting | SEP 2013 – DEC 2014
Life Underground
Project Summary
Our multi-disciplinary team from USC, Caltech, JPL, DRI, RPI, and now also Northwestern is developing and employing field, laboratory, and modeling approaches aimed at detecting and characterizing microbial life in the subsurface—the intraterrestrials. We posit that if life exists, or ever existed, on Mars or other planetary body in our solar system, evidence thereof would most likely be found in the subsurface. This study takes advantage of unique opportunities to explore the subsurface ecosystems on Earth through boreholes, mine shafts, sediment coring, marine vents and seeps, and deeply-sourced springs. Access to the subsurface—both continental and marine—and broad characterization of the rocks, fluids, and microbial inhabitants is central to this study. Our focused research themes require subsurface samples for laboratory and in situ experiments. Specifically, we are carrying out in situ life detection, culturing and isolation of heretofore unknown intraterrestrial archaea and bacteria using numerous novel and traditional techniques, and incorporating new and existing data into regional and global metabolic energy models.
Project Progress
The centerpiece of our research effort is a set of promising and geologically representative deep subsurface sites on Earth, both continental and marine. These provide samples and opportunities around which we are conducting several comprehensive, interdisciplinary, coordinated, and complementary research efforts. Here, we provide brief summaries of the main accomplishments during this reporting period for four major research themes: (A) access to the subsurface and broad characterization; (B) in-situ life detection and characterization, including mission relevance; (C) guided cultivation of intraterrestrials; and (D) energy flow and metabolic modeling.
A. Access to the Subsurface and Broad Characterization.
In 2014, the Life Underground project built upon our 2013 work at the Sanford Underground Research Facility (SURF) in South Dakota and boreholes in and around Death Valley; we also continued ongoing work at an ophiolite site in northern California called ‘the Cedars’. In the marine realm, members of our team participated in IODP Drilling Expedition 337 to the Shimokita Peninsula (Japan) in the northwestern Pacific, and collaborations continued with the NSF-funded Center for Dark Energy Biosphere Investigations (C-DEBI), which focuses on bio-sphere of the marine subseafloor (see Section 9). For all sites—continental and marine—collections of standardized samples and metadata continued, including: temperature, pH, oxidation-reduction potential, dissolved oxygen, total dissolved solids, aqueous and nutrient chemistry analysis, metal speciation, dissolved gas compositional and isotopic characterizations (dC and d2D), sulfur and water isotopes (e.g. d34S, and d2D/d18O), organic constituents, electron micros-copy, microbiology, planktonic cell abundance, cultivations, single cell genomics, and filters for DNA and lipids.
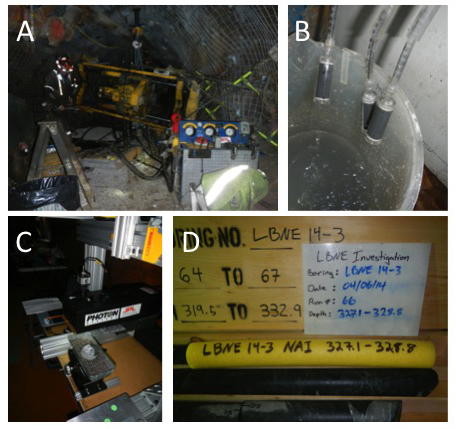
SURF: Numerous members of the Life Underground team (investigators, students, post-docs, and technicians) are safety-certified to work at SURF, and several projects were continued or initiated in 2014. A keystone activity was our participation in an underground drilling project on the 4850 level, (1,478 m depth). Four sub-horizontal holes (145-343 m in length) were drilled by engineering contractors to obtain test cores for large cavity construction. We were allowed 3.2 m of the 168 m core (LBNE-14-3), selected for its remoteness from historic mining and proximity to potentially water-bearing Tertiary dykes. Unfortunately, none of the LBNE coreholes produced significant water. Subsamples of the phyllite/rhyolite cores were processed underground for preservation of microbial DNA (freezing), cultivation, and deep UV scanning. A new experiment was also started at the 4850 level at legacy hole “3A”, located near electrical and data service. Here, In situ electrode-assisted cultivation of subsurface microbes is being conducted for the first time (see Section 3C). The next phase of work at SURF will involve proposals to develop a distributed, kilometer-scale, 3D microbial observatory using select legacy holes from the surface to the 4850 level.
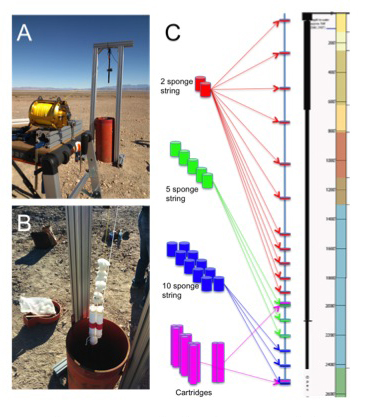
Great Basin: One of our priority (“Tier-1”) sites is a borehole near Death Valley known as BLM-1. Drilled in 2005 to 883 m, this hole taps ~60oC water in an anoxic carbonate aquifer. In 2014, a memorandum of understanding between the National Park Service, USC and DRI established BLM-1 as a deep life observatory for the NAI. Re-entry is being conducted as a staged process, initiated with downhole substrate incubations in November of 2014. Substrates included sponges, mineral coupons, glass wool, and steel casing material. The next phase will involve further sampling and comprehensive logging.
The Cedars: This site in northern California is part of an ophiolite complex characterized by peridotite serpentinization that yields fluids of very high pH, low redox potential, and low ionic strength. The Cedars has been under continuous study by Co-I Nealson and colleagues for a number of years, and extensive cultivation efforts and community metagenomic and meta-transcriptomic analysis continued throughout 2014 in both the shallow and deep-fed springs.
Shimokita Peninsula: In July-Sept, 2012, Life Underground graduate student Elizabeth Trem-bath-Reichert (Caltech) and collaborators Fumio Inagaki, Yuki Morono and Hiro Imachi participated in IODP Drilling Expedition 337 to the Shimokita Peninsula. This expedition broke the record for deepest riser drilling on the scientific drillship Chikyu, drilling to over 2.2 km and collecting samples from shale beds, sandstone and coal. At sea, 888 stable isotope incubations were established, and Trembath-Reichert and Japanese collaborators have been analyzing these incubations for signs of microbial respiration and growth.
B. In-situ Life Detection and Characterization, and Mission Relevance.
In this theme, we aim to detect and characterize in situ life based on spatial distribution of organics (e.g., microorganisms), minerals, elements and isotopes, in the context of textural features, from the macro to the micro scale. The team uses a number of optical techniques ranging the electromagnetic spectrum from the deep UV (<250 nm) to the IR using an array of spectral phenomena such as absorption, Raman scattering, fluorescence, and reflectance for noninvasive means of assessing macro to micro structure, organics, minerals, and elements. These methods help refine samples to smaller targets of interest for analysis by more “invasive” but chemically specific analyses including phylogenetic identification of microorganisms and community structure to elemental/isotopic analysis using techniques such as nanoSIMS.
As described above we have several sets of samples currently incubating at SURF and BLM-1 and have recently recovered a suite of samples from BLM-1 These samples, specifically mineral coupons, are now being fed directly into the macro-to-micro life detection and characterization pipeline. Over the last year advancements have been made allowing for samples to be moved between instruments and analyses while maintaining spatial correlations. We are currently in the process of combining these data sets. This has required modifications to tried-and-tested methods as well as the writing of new analyses software to handle data from various instruments over a series of spatial scales. The presence of the NAI team during the LBNE coring allowed us to collect spectroscopic data in the field before samples were archived for laboratory analyses. Core samples were scanned by the MOSAIC deep-UV scanner minutes after extraction.
In 2014, we focused on development of the sample analysis process using a series of lab standards, samples incubated in local field sites operating as “windows” to the subsurface, and subsurface materials already on hand. While this is an ongoing process, initial results demonstrate the uniqueness of the techniques being used. One technique for field and lab samples, deep (UV Raman/fluorescence) has been under development for the past 16 years as a means to search for organics and habitable regions without the need for sample handling. This technique was recently selected for the upcoming Mars 2020 mission as the flight instrument SHERLOC (Scanning Habitable Environment with Raman and Luminescence for Organics and Chemicals) where Co-Is/Collaborators of the Life Underground team are SHELROC Deputy PI (R. Bhartia), Co-Is (K. Nealson and B. Ehlmann). In a similar manner to the stated goals of Mars 2020, the Life Underground team is using the deep UV Raman/fluorescence as a means to detect and map organics and microbes living in rock such that we can focus detailed mineralogical, structural, and elemental/isotopic techniques. In addition, and in line with the M2020 payload, the Life Underground team is combining multispectral, multispatial, and temporal methods to enable “correla-tive imaging” as a means to better understand the living subsurface communities. The deep UV instruments are being tested for both laboratory analyses and in situ use at the Life Underground field sites.
The Life underground team was also presented with a sampling expedition of opportunity. A newly planned underground physics laboratory, the Long Baseline Neutrino Observatory, required rock core samples be drilled on the 4850’ level as part of a geotechnical assessment prior to construction of the underground cavern. Several of the NAI team members were invited to participate in drilling operations and were present during the coring (Figure 1). The team was on location at SURF as the cores were extracted and performed onsite analyses, including scanning of the cores with MOSAIC, a Deep UV- Fluorescence mapping instrument (a processor to the SHERLOC instrument described below). Sections of core were also preserved on site and returned to USC and JPL for downstream analyses, including DNA extraction and sequencing, microscopy, and higher resolution Deep UV scanning. These analyses are ongoing.
Characterization of the SURF boreholes continued this year with the installation of two long-term experiments plumbed directly into two boreholes. The first of theseconsists of a series of cartridges filled with crushed native minerals collected previously from the 4850L (Figure 2). Along with the crushed minerals several polished coupons of the same minerals were included. These mineral coupons were characterized prior to deployment with the Deep-UV Raman and Fluorescence instruments and Electron Microscopy with elemental analysis. These T=0 data are helping us to design and streamline operations and data management. These coupons represent ‘simplified’, mineralonly controls that we are using to verify the macro-to-micro analysis pipe-line for all samples collected in the future. The SURF cartridges will be collected around March/April 2015 and will be processed thought the entire pipeline (i.e. Photography, Deep-UV Fluorescence/Raman, FISH-Fluorescence Microscopy, Electron Microscopy and nanoSIMS). Efforts have been taken so that features on the mineral chips are registered allowing for direct comparisons of specific features from the uninoculated coupons.
Efforts at our Tier 1 site in the desert (borehole BLM-1) also continued. In November 2014 we successfully deployed a large suite of samples in the borehole. A sample string consisting of sponges and cartridges (Figure 3), similar to those deployed at SURF, were placed into the bore-hole and allowed to incubate until February 2015. The cartridges were filled with representative minerals as well as minerals extracted during the drilling of the BLM-1 borehole. Polished coupons of the minerals will be subjected to the macro-to-micro sample pipeline (i.e. Spectroscopy → Microscopy → NanoSIMS → Molecular biology). As mentioned, these samples were just recovered by team members from JPL, Caltech and DRI and have been distributed to the labs for further analysis. After recovery of the initial sample string another identical string was lowered into the hole and is currently incubating until ~May 2015. This current incubation time however may be extended pending the results from the first sample string. The samples thus far recovered from BLM-1 have been distributed as highlighted below:
Ongoing analyses of BLM-1 sample string:
o DRI → Community analysis and culturing
o USC → Culturing (hanging sponge reactor)
o JPL → UV/Visible light spectroscopy (Raman and Fluorescence – SHERLOC)
o Caltech → Isotope labeled culturing, Microscopy, nanoSIMS
o Future samples with other NAI nodes
Work at the deeply-sourced springs at ‘the Cedars’ also continued in 2014. After an in-depth description of the study site (Morrill et al., 2013), a detailed analysis of the in situ microbial populations (Suzuki et al., 2013), new methods for population analysis using metatranscriptomics (Ishii et al., 2013a), and the detection of genes activated by changes in surface electrochemical charge (Ishii et al., 2013b), we finished some detailed studies of the physiology of bacteria isolated from the Cedars (Suzuki et al., 2014). In addition, we deployed and recovered mineral colonization experiments. The recovered mineral coupons were rescanned with the deep-UV Raman and fluorescence systems to map the extent of the microbial colonization. The DNA on the samples was then extracted and is currently undergoing sequencing to determine the microbial community structure. A suite of carbonate samples was also collected from ‘the Cedars’ spanning a range of ages (e.g. 1-2 years, 100’s of years and 1000’s of years old). These samples are being scanned with the Deep-UV instruments to map the minerals and organics entrained within the mineral matrix. These samples will be used to look at the short-term (i.e. <1000 years) preservation of microbes in these carbonates.
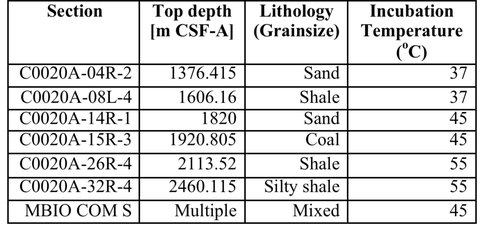
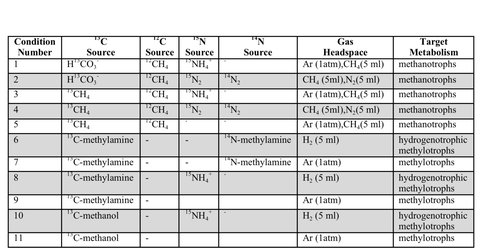
On the marine side of the Life Underground project, Caltech graduate students Trembath-Reichert, Case, and Marlow have been involved in sample collection and experimental set up for deep subseafloor sites. Trembath-Reichert sailed on IODP Expedition 337 (Shimokita Coalbed) and worked with NAI collaborating partners from JAMSTEC in Japan (Inagaki and Moreno) to prepare stable isotope incubations (13C, 15N and D/H) from 4 different lithologies ranging from sandstone to coal (Table 1, Table 2, and Figure 4). The chosen study area for Expedition 337 had a high probability to retrieve core from deeply buried coal beds of low thermal maturity, increasing the potential to find deep life by exploring an environment that could utilize coal deposits for carbon and potentially other essential nutrients. Analyses of 13C enrichment in the DIC pool and CH4 production after 12 months indicates active microbial respiration in the >2 km coal bed samples with C1 carbon substrates. Samples have recently been harvested and a subset of these will be run through the deep UV Raman, FISH and nanoSIMS analysis pipeline to begin to assess microbial colonization patterns and activity across a range of lithologies and habitats. Preliminary Deep UV-Raman analysis of the coal from Shimokita is being used to map microbial features associated with the coal as well as a geothermometer showing the maturity of the organics within. In 2015, Trembath-Reichert will be visiting the laboratory of international collaborator Fumio Inagaki (JAMSTEC) to prepare samples for single cell genomic sequencing and na-noSIMS from the Shimokita incubations maintained at Caltech and Japan.
3C. Guided Cultivation of Intraterrestrials.
Characterization of the subsurface biosphere is hindered because the majority of the resident microorganisms appear to be ‘unculturable’ using traditional growth techniques that rely on batch experiments or well-stirred vessels. We are focused on developing and harnessing new cultivation techniques that go beyond these traditional limitations. Our approaches mimic the complex interactions and energetic gradients present in the NAI field sites in order to reveal the full diversity of subsurface microorganisms. During this reporting period, our progress on the cultivation front was as follows:
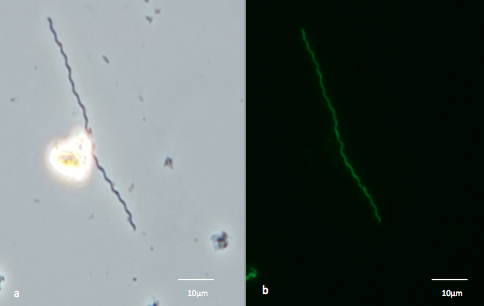
Down-flow Hanging Sponge Reactors for investigating microbial communities in porous media. Graduate student Lily Momper in the Amend lab at USC constructed, tested, and operated down-flow hanging sponge (DHS) bioreactors under two conditions for enrichment of (i) Metal (Fe/Mn) reducing bacteria and (ii) Mn oxidizing bacteria or Thaumarchaeota under microoxic conditions (Amend Lab). The DHS technology was transferred to our team members (Amend, Orphan) from our JAMSTEC collaborator (Dr. Hiro Imachi). These bioreactors were inoculated from our preliminary sampling projects at Nevares Deep Well 2 and SURF. Preliminary results indicated successful enrichment, as evidenced by biomass measurements, 16S rRNA analysis, and microscopy. From the Nevares Deep Well 2 enrichments, two novel bacteria have been isolated. One falls within the genus Thermincola, a group of chemolithoautotrophis known to reduce ferric iron with hydrogen as an electron donor using carbon dioxide as the sole carbon source. The other bacterium is a novel member of the family Spirochaetaceae (Figure 5). From SURF, we enriched a novel member of the phylum Thaumarchaeota; isolation attempts are underway, and lipid characterization will be conducted in collaboration with Roger Summons and the MIT CAN-6 team.
At Caltech, manganese and iron-oxide amended DHS bioreactors inoculated with marine methane seep sediment stimulated the growth of a novel bacterial lineage affiliated with the Tenner-icutes, a group which is predominately associated with animal and plant pathogens (e.g. mycoplasma). Two genomes of this new bacterial group, previously known only from environmental 16S rRNA sequences, were reconstructed from metagenomic data. The predicted metabolism and physiology of this organism was used to design enrichment media. These efforts have recently resulted in a successful enrichment culture of this organism (tentatively named Izimaplasma) and work is ongoing to purify this organism for further physiological characterization.
Electrode Cultivation. It has been suggested that extracellular electron transport may be an important mode of metabolism in energy-limited environments, including the subsurface. This has expanded to using solid surfaces (minerals and charged electrodes) as either electron acceptors for respiration or as electron donors (energy sources). During the first year of our project, the El-Naggar lab led the development of the first electrode cultivation reactor targeting subsurface microorganisms, and we used this platform to enrich for metalreducing bacteria at four redox con-ditions (50 mV, 150 mV, 250 mV, and 350 mV vs. Ag/AgCl) from our preliminary sampling at Nevares Deep Well 2 (Death Valley, CA). The enrichment was successfully completed in 2014, and the communities were assessed using 16S rRNA gene analysis (manuscript in preparation). While alternate metabolisms from the dominant families present (e.g. denitrification by Comamonadaceae and Rhodocyclaceae) are well characterized, our electrode enrichments suggest that these microbes are also capable of extracellular electron transfer to additional insoluble electron acceptors. We are currently isolating pure strains for additional mechanistic studies. In addition, members of the Nealson lab used new electrode cultivation techniques to isolate microbes from marine sediments (Rowe et al., 2015).
In situ Subsurface Cultivation. In addition to our laboratory efforts, we have designed, constructed, and deployed the first experimental system of its kind: an electrode cultivation bioreactor at 4850 ft below the surface, fed with water from legacy hole 3A at SURF in South Dakota. The system is now full operational (Figure 6), and is enriching for microbes at 4 different redox conditions mimicking specific metabolisms predicted by our team’s recent modeling effort (Osburn et al., 2014).
Components and mechanisms of extracellular charge transfer in subsurface microbes. Post-doc Anja Bauermeister in the Gorby lab developed electrocultivation capabilities at RPI to support a wide range of subsurface metabolisms, including for dissimilatory iron-reducing and iron-oxidizing bacteria operating at pH values ranging from 2.5 to 12. These devices not only provide the ideal platform to enrich for and identify novel biological components involved in extracellular electron transfer, but they are proving invaluable for exploring a full spectrum of biogenic minerals that may directly or indirectly contribute to charge transfer in subsurface ecosystems under low energy conditions. In 2014, Bauermeister collected sediment samples from salt marshes and freshwater systems and screened them for “electric cable bacteria” with the objective to study the mode of electron transport along the filaments and its impact on sediment mineralogy. These efforts are ongoing, as most samples possibly containing cable bacteria were overgrown by Beggiatoa.
A second project focused on ‘the Cedars’ site, a present-day serpentinization site in northern California with discharges of ultrabasic groundwater. The objective there is to isolate, culture, and characterize microbial strains from the deep groundwater springs with a focus on microbial autotrophy and interaction of microorganisms with minerals such as magnetite and olivine. Liquid enrichment cultures on different electron donors and acceptors have shown stable growth over several transfers. Agarose gradients will be set up anaerobically and analyzed by fluorescence microscopy, pH depth profiling, and DNA extraction, as well as electron microscopy. These agarose gradient chambers will be instrumented with electrodes poised at specific potentials to encourage growth of bacteria on solid electron acceptors or donors.
D. Energy Flow and Metabolic Modeling.
Several projects in the energy flow and metabolic modeling theme made significant advances, resulting in published and submitted papers, as well as manuscripts in preparation. These projects include global models of organic carbon in marine sediments, the power limits of microbial life, energy calculations of chemolithotrophic reactions in several environments, and a modeling effort of microbial limitations going back to the time of life’s emergence on Earth more than 3.5 billion years ago.
In LaRowe et al. (in preparation), we addressed microbial transformations of organic matter in marine sediments through time. We generated a quantitative a quantitative description of global marine sediments as a habitat for microorganisms. In order to accomplish this, a number of global data sets were combined with a reactive continuum model describing organic matter degradation to quantify the physical and chemical characteristics of marine sediments and the distribution of organic matter in it. In addition to new estimates for the global volume of marine sediments (3.038 × 108 km3) and sedimentary pore water (8.103 × 107 km3), results from this study show how porosity and temperature are distributed in three dimensions. Furthermore, a reactive continuum model has been used to map the distribution of organic matter in marine sediments in three dimensions for sediments throughout the Pleistocene. Results indicated that, the rate of organic matter degradation in Holocene sediments is 361 Tg C yr-1, while the rate in the much more voluminous Pleistocene-aged sediments is only 11 Tg C yr-1. These results inform on the role of microbial activity in shaping diagenesis, influencing the carbon cycle, and the extent of life in the subsurface.
In LaRowe and Amend (in review), we used existing data on power requirements for life and models to constrain the minimum flux of energy needed by microbial cells. A bioenergetics model was applied to a well-characterized marine sedimentary environment in order to quantify the amount of power organisms use in an ultralow-energy setting, representative of many subsurface environments. In particular, a direct link between the amount of power supplied to this environment and the amount of biomass found in it is shown. The power supply resulting from the aerobic degradation of particulate organic carbon (POC) at IODP site U1365 in the South Pacific Gyre is between ~ 10-11 to 10-17 W cm-3. The requisite rates of POC degradation are calculated using the Reactive Continuum Model while Gibbs energies are a function of the local geochemistry. Although laboratory-determined values of maintenance power for single cells do a poor job of calculating the amount of biomass in U1365 sediments, the number of cells per cm-3 can be well-captured using a maintenance power, 190 zW cell-1, two orders of magnitude lower than that reported in the literature. In addition, cell counts and power supplies were combined to determine that, on average, the microorganisms site U1365 require from 44 – 340 zW cell-1, depending on the depth below the seafloor. Furthermore, an analysis of the absolute minimum power requirement for a single cell to remain viable has been determined to be on the order of 1 zW cell-1.
In Osburn et al. (2014) and in Price et al. (in review), we calculated reaction energetics for chemolithotrophs in the deep fracture fluids at SURF and in a shallow-sea hydrothermal vent environment at Panarea Island (Italy), respectively. At SURF, geochemical data, energetic modeling, and DNA sequencing were combined with principle component analysis to describe this deep (down to 8100 ft below surface), terrestrial environment. Gibbs energy calculations reveal an abundance of energy for microorganisms from the oxidation of sulfur, iron, nitrogen, methane, and manganese. Extrapolated across the mine footprint, these data suggest a complex spatial mosaic of subsurface primary productivity that is in good agreement with predicted energy yields. Notably, we report Gibbs energy normalized both per mole of reaction and per kg fluid (energy density) and find the later to be more consistent with observed physiologies and environmental conditions. Further application of this approach will significantly expand our understanding of the deep terrestrial biosphere. At Panarea in order to investigate subsurface processes in shallow-sea hydrothermal systems, and to determine how these physical and chemical parameters influence the metabolic potential of the microbial communities, we characterized three shallow vents. Vent fluids, pore fluids, and gases were sampled and analyzed for majore and minor elements, redox-sensitive compounds, and free gas compositions to evaluate the catabolic potential of 61 inorganic redox reactions for in situ microbial communities. Gibbs energies (ΔGr) of redox reactions that couple potential terminal electron acceptors (O2, NO3-, MnIV, FeIII, SO42-, S0, CO2,) with potential electron donors (H2, NH4+, Fe2+, Mn2+, H2S, CH4) were evaluated at in situ temperatures and compositions for each site and by fluid type. When Gibbs energies of reaction are normalized per kilogram of hydrothermal fluid, sulfur oxidation reactions are the most exergonic, while the oxidation of Fe2+, NH4+, CH4, and Mn2+ are moderately energy yielding. The energetics calculations indicate that the most robust microbial communities in the Panarea hot springs combine H2S from deep water-rock-gas interactions with O2 that is entrained via seawater mixing to fuel their activities, regardless of site location or fluid type.
In Kempes et al. (in preparation), we carried out some modeling on microbial cell size, composition, and evolutionary limitations. We present a theory, which we verify using compiled data, for the changes in cellular composition across the entire range of bacteria cell sizes. Our analysis spans a huge diversity of species and 4 orders of magnitude in body size. We show that the interconnection between energetic, physical, informational (genomic), chemical, and temporal limitations constrain the upper and lower boundaries of bacterial size and define the evolutionary flexibility for bacteria between these two bounds.
References
Belcher, WR, Bedinger, MS, Back, JT, and Sweetkind, DS (2009) Interbasin flow in the Great Basin with special reference to the southern Funeral Mountains and the source of Furnace Creek springs, Death Valley, California, U.S., J. Hydrol. , 369, 30-43.
Belcher, WR, and Sweetkind, DS, eds. ( 2010) Death Valley regional groundwater flow system, Nevada and California—Hydrogeologic framework and transient groundwater flow model: U.S. Geological Survey Professional Paper 1711, 398 pp.
Collier, M (1990) An Introduction to the Geology of Death Valley. Death Valley, California: Death Valley Natural History Association. LCN 90-081612.
Harris, AGT, and Tuttle, SD (1997) Geology of National Parks (5th ed.). Iowa: Kendall/Hunt Publishing. ISBN 0-7872-5353-7.
Ishii, S, Suzuki, S, Norden-Krichmar, TM, Tenney, A, Chain, PSG, Scholz, MB, Nealson, KH, and Bretschger, O (2013a) A novel metatranscriptomic approach to identify gene ex-pression dynamics during extracellular electron transfer. Nature Communications, (4:1601) DOI: 10.1038/ncomms2615
Ishii, S, Suzuki,S, Norden-Krichmar, TM, Phan, T, Wanger, G, Nealson, KH, Sekiguchi, Y, Gorby, YA, and Bretschger, O (2013b) Microbial population and functional dynamics asso-ciat-ed with surface potential and carbon metabolism, The ISME J. (in press)
Kempes, CP, Wang, L, Amend, JP, Doyle, J, Hoehler, T, Body size, cellular composition, and the evolutionary limitations of bacteria. Proceedings of the National Academy of Sciences.
LaRowe, DE and Amend, JP, Power limits of microbial life. Frontiers in Microbiology (in re-view).
LaRowe, DE, Burwicz, E, Arndt, S, Dale, AW, Amend, JP, Global marine sediments as microbi-al habitats: Distribution of water, organic carbon, temperature, and pore space in marine sed-iments.
Morrill, PL, Kuenen, JG, Johnson, OJ. et al. (2013) Geochemistry and geobiology of a present-day serpentinization site in California: The Cedars, Geochimica et Cosmochimica Acta, 109, 222-240.
Osburn, MR, Momper, LM, LaRowe, DE, Amend, JP (2014), Chemolithoautotrophy in the con-tinental deep subsurface: Sanford Underground Research Facility (SURF), USA, Frontiers in Extreme Microbiology, doi:10.3389/fmicb.2014.00610.
Price, RE, LaRowe, DE, Italiano, F, Savov, I, Pichler, T, Amend, JP, Subsurface hydrothermal processes and the bioenergetics of chemolithoautotrophy at the shallow-sea vents off Panarea Island (Italy). Chemical Geology (in review).
Rowe, A, Chellamuthu, P, Lam, B, Okamoto, A, and Nealson, K (2015) Marine sediments mi-crobes capable of electrode oxidation as a surrogate for lithotrophic insoluble substrate me-tabolism. Frontiers in Microbiology. DOI:10.3389/fmicb.2014.00784
Suzuki, S, Ishii, S, Wu, A, Cheung, A, Wanger, G, Tenney, A, Kuenen, JG, and Nealson, KH (2013) The Cedars actively serpentinizing ecosystem: Comparison of microbial diversity in the ultra-basic, ultra-reducing and low salinity serpentinizing ecosystem, PNAS 110, 15336-15341
Suzuki, S, Kuenen, JG, Schipper, K et al. (2014) Physiological and genomic features of highly alkaliphilic hydrogen-utilizing Betaproteobacteria from a continental serpentinizing site, Na-ture Communications, 5.
Winograd, IJ, Fridrich, CJ, Sweetkind, D, Belcher, WR, and Thomas, JM (2005) Comment on “testing the interbasin flow hypothesis at Death Valley, California”,, EOS, Transactions of the American Geophysical Union, 86, 295–296.

Publications
-
Gilhooly, W. P., Fike, D. A., Druschel, G. K., Kafantaris, F-C., Price, R. E., & Amend, J. P. (2014). Sulfur and oxygen isotope insights into sulfur cycling in shallow-sea hydrothermal vents, Milos, Greece. Geochemical Transactions, 15(1), None. doi:10.1186/s12932-014-0012-y
-
LaRowe, D. E., Dale, A. W., Aguilera, D. R., L’Heureux, I., Amend, J. P., & Regnier, P. (2014). Modeling microbial reaction rates in a submarine hydrothermal vent chimney wall. Geochimica et Cosmochimica Acta, 124, 72–97. doi:10.1016/j.gca.2013.09.005
-
Marlow, J. J., LaRowe, D. E., Ehlmann, B. L., Amend, J. P., & Orphan, V. J. (2014). The Potential for Biologically Catalyzed Anaerobic Methane Oxidation on Ancient Mars. Astrobiology, 14(4), 292–307. doi:10.1089/ast.2013.1078
-
Marlow, J. J., Steele, J. A., Case, D. H., Connon, S. A., Levin, L. A., & Orphan, V. J. (2014). Microbial abundance and diversity patterns associated with sediments and carbonates from the methane seep environments of Hydrate Ridge, OR. Frontiers in Marine Science, 1. doi:10.3389/fmars.2014.00044
-
Marlow, J. J., Steele, J. A., Ziebis, W., Thurber, A. R., Levin, L. A., & Orphan, V. J. (2014). Carbonate-hosted methanotrophy represents an unrecognized methane sink in the deep sea. Nat Comms, 5, 5094. doi:10.1038/ncomms6094
-
Meyer-Dombard, D. A. R., & Amend, J. P. (2014). Geochemistry and microbial ecology in alkaline hot springs of Ambitle Island, Papua New Guinea. Extremophiles, 18(4), 763–778. doi:10.1007/s00792-014-0657-6
-
Okamoto, A., Hashimoto, K., & Nealson, K. H. (2014). Flavin Redox Bifurcation as a Mechanism for Controlling the Direction of Electron Flow during Extracellular Electron Transfer. Angew. Chem. Int. Ed., 53(41), 10988–10991. doi:10.1002/anie.201407004
-
Okamoto, A., Kalathil, S., Deng, X., Hashimoto, K., Nakamura, R., & Nealson, K. H. (2014). Cell-secreted Flavins Bound to Membrane Cytochromes Dictate Electron Transfer Reactions to Surfaces with Diverse Charge and pH. Scientific Reports, 4. doi:10.1038/srep05628
-
Okamoto, A., Nakamura, R., Nealson, K. H., & Hashimoto, K. (2014). Bound Flavin Model Suggests Similar Electron-Transfer Mechanisms in Shewanella and Geobacter. CHEMELECTROCHEM, 1(11), 1808–1812. doi:10.1002/celc.201402151
-
Orcutt, B. N., & Edwards, K. J. (2014). Life in the Ocean Crust. Developments in Marine Geology, None, 175–196. doi:10.1016/b978-0-444-62617-2.00007-4
-
Osburn, M. R., LaRowe, D. E., Momper, L. M., & Amend, J. P. (2014). Chemolithotrophy in the continental deep subsurface: Sanford Underground Research Facility (SURF), USA. Frontiers in Microbiology, 5. doi:10.3389/fmicb.2014.00610
-
Rowe, A. R., Chellamuthu, P., Lam, B., Okamoto, A., & Nealson, K. H. (2015). Marine sediments microbes capable of electrode oxidation as a surrogate for lithotrophic insoluble substrate metabolism. Frontiers in Microbiology, 5. doi:10.3389/fmicb.2014.00784
-
Suzuki, S., Kuenen, J. G., Schipper, K., Van Der Velde, S., Ishii, S. i., Wu, A., … Nealson, K. H. (2014). Physiological and genomic features of highly alkaliphilic hydrogen-utilizing Betaproteobacteria from a continental serpentinizing site. Nat Comms, 5. doi:10.1038/ncomms4900
-
Takai, K., Nakamura, K., LaRowe, D., & Amend, J. P. (2014). Life at Subseafloor Extremes. Developments in Marine Geology, None, 149–174. doi:10.1016/b978-0-444-62617-2.00006-2
-
Torres, M. A., West, A. J., & Nealson, K. (2014). Microbial Acceleration of Olivine Dissolution via Siderophore Production. Procedia Earth and Planetary Science, 10, 118–122. doi:10.1016/j.proeps.2014.08.041
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Jan Amend
Project Investigator
Rohit Bhartia
Co-Investigator
Moh El-Naggar
Co-Investigator
Tracy Fullerton
Co-Investigator
Yuri Gorby
Co-Investigator
Duane Moser
Co-Investigator
Kenneth Nealson
Co-Investigator
Victoria Orphan
Co-Investigator
Magdalena Osburn
Co-Investigator
Holly Willis
Co-Investigator
William Abbey
Collaborator
Anja Bauermeister
Collaborator
Lina Bird
Collaborator
David Case
Collaborator
Gray Chadwick
Collaborator
Stephanie Connon
Collaborator
Bethany Ehlmann
Collaborator
Scott Hamilton-Brehm
Collaborator
Fumio Inagaki
Collaborator
Yamini Jangir
Collaborator
Chris Kempes
Collaborator
Brittany Kruger
Collaborator
J. Gijs Kuenen
Collaborator
Doug LaRowe
Collaborator
Bonita Lam
Collaborator
Lucas Lis
Collaborator
Jeffrey Marlow
Collaborator
Urbashi Mitra
Collaborator
Lily Momper
Collaborator
Penny Morrill
Collaborator
Sean Mullin
Collaborator
Akihira Okamoto
Collaborator
Veronica Paez
Collaborator
Sahand Pirbadian
Collaborator
Brandi Reese
Collaborator
Nerissa Rivera
Collaborator
Alberto Robador
Collaborator
Tyler Roche
Collaborator
Karyn Rogers
Collaborator
Annette Rowe
Collaborator
Joshua Sackett
Collaborator
Pratixa Savalia
Collaborator
Shakher Sijapati
Collaborator
Shino Suzuki
Collaborator
Shino Suzuki
Collaborator
Elizabeth Trembath-Reichert
Collaborator
Greg Wanger
Collaborator
Laura Zinke
Collaborator
-
RELATED OBJECTIVES:
Objective 2.1
Mars exploration.
Objective 2.2
Outer Solar System exploration
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.3
Origins of energy transduction
Objective 4.1
Earth's early biosphere.
Objective 5.1
Environment-dependent, molecular evolution in microorganisms
Objective 5.2
Co-evolution of microbial communities
Objective 5.3
Biochemical adaptation to extreme environments
Objective 6.1
Effects of environmental changes on microbial ecosystems
Objective 6.2
Adaptation and evolution of life beyond Earth
Objective 7.2
Biosignatures to be sought in nearby planetary systems