2014 Annual Science Report
 NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2013 – DEC 2014
NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2013 – DEC 2014
Titan as a Prebiotic Chemical System - Willacy
Project Summary
To develop a comprehensive model of the chemistry in Titan’s atmosphere including condensation of molecules onto grains and sublimation back to the gas, and exchange between the atmosphere and surface.
Project Progress
The KINETICS code is a couple chemistry/dynamics code that has been extensively developed over the past 5 years to provide a new, sophisticated description of the organic chemistry in Titan’s atmosphere. The major accomplishments over the lifetime of the NAI have included
1. Parallelization to enable efficient computation of 2-D atmospheric models
2. Addition of a numerically stable description of the condensation and sublimation processes (including exchange with the surface) and the application of this to the problem of matching the observations of H2 in Titan’s atmosphere.
3. Inclusion of observationally determined aerosol characteristics into the condensation and radiation modules.
4. Updating of the reaction database used to model the chemistry in Titan’s atmosphere including a vastly expanded description of the organic chemistry.
5. Updating of the photodissociation cross-sections used in the model
A) Condensation and sublimation processes:
In general, models of sublimation and condensation in Titan’s atmosphere have used a method that can become numerically unstable. We have implemented a numerically stable method of determining the gas-grain interactions which can be easily incorporated into the chemical kinetics equations. The condensation rate is determined by the collision rate of a molecule with the grain surface, and the sublimation rate from the saturated vapor pressure. The net flow of a molecule x onto a grain surface is therefore given by
dn/dt=S_x σv_x (n_sat (x)θ- n) molecules/s
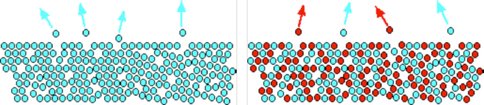
where SX = sticking coefficient, σ = surface area of grain, ng = number density of grains, vX = gas phase velocity, θ= surface coverage, and nsat(X) is the saturation density calculated from the saturated vapor pressure. The expression is positive for net condensation and negative for net sublimation. This method is similar to that used in Earth’s atmosphere for the kinetic regime and is valid throughout the majority of Titan’s atmosphere, above an altitude of 65km. Below this altitude, our method overestimates the condensation rate by a factor of ~ 1.6. The inclusion of the surface coverage, θ, in the sublimation means that we can take into account the reduced desorption from mixed ices. This is illustrated in Figure 1.
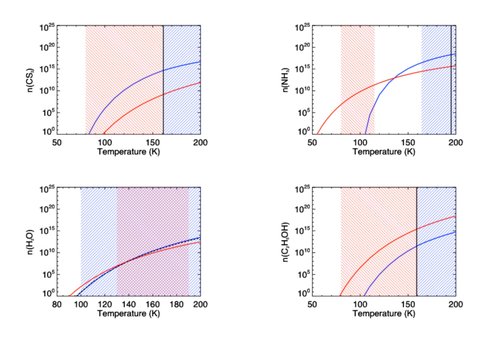
In addition to using saturated vapor pressures (as described above), sublimation rates can also be calculated using the binding energy, EB, of a molecule to the grain surface. In principle the methods should be equivalent and result in the same predicted gas phase abundances. We compared the predicted gas phase abundances for several species for which both binding energy and saturation vapor pressure data have been measured (Figure 2) and found large discrepancies in some cases. The reasons for this are not clear. We have begun collaboration with Dr. Wendy Brown (University of Sussex) to further explore possible reasons for the lack of agreement between the two types of sublimation calculation
B. H2 in Titan’s atmosphere.
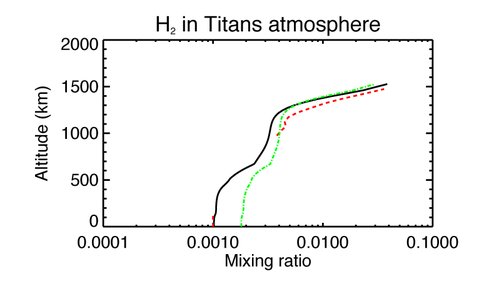
H2 is the third most abundant molecule in Titan’s atmosphere. It has been detected at high altitudes and close to the surface of the moon. The difficulty of some models in reproducing both these sets of data has led to the suggestion that the downward flux of H2 into the Titan surface could indicate the presence of methanogenic life on the surface (Strobel 2010 Icarus 208, 878).
Figure 3 shows the predicted H2 abundance from our model. The green dashed line is for a gas phase only model and reproduces the factor-of-two discrepancy between matching the upper and lower atmospheric observations found by Strobel (2010). H2 is too volatile to condense in Titan’s atmosphere. However, other molecules that provide sources of H2 are condensed e.g. hydrocarbons. Adding gas-grain interactions allows us to match both sets of observations without needing to invoke the presence of methanogenic life. We are currently testing the sensitivity of our results to three crucial processes:
1. Transport between the stratosphere and troposphere as parameterized by the eddy diffusion coefficient, which has been fine-tuned to reproduce the Cassini data for many chemical species (Li et al. 2014, Planet. Space Sci. 104, 48 ).
2. Rate of production of H2 from the dissociation of hydrocarbons that are affected by condensation.
3. The time constant for H2 to reach a quasi-steady state on Titan.
We will be writing up our results for publication shortly.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Mark Allen
Project Investigator
Karen Willacy
Co-Investigator
Yuk Yung
Co-Investigator
-
RELATED OBJECTIVES:
Objective 2.2
Outer Solar System exploration
Objective 3.1
Sources of prebiotic materials and catalysts