2014 Annual Science Report
 NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2013 – DEC 2014
NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2013 – DEC 2014
Co-Crystals on the Surface of Titan
Project Summary
We have discovered that benzene and ethane form a co-crystalline inclusion compound at Titan surface temperatures and pressures. Co-crystals of other organic compounds could be common on Titan’s surface. These results can help explain the release of ethane observed at the Huygens landing site, and point to a new type of surface material that may have significant impact on Titan surface chemistry and geology.
Project Progress
Titan is the only body in the Solar System aside from Earth that has standing liquid on its surface. Due to the low surface temperatures (90-95 K), this liquid phase is comprised of hydrocarbons, mostly methane and ethane. Active photochemistry in the atmosphere due to solar radiation and energy from Saturn’s magnetosphere generates a plethora of organic molecules, from simple molecules to compounds >10,000 Da. These species are ultimately deposited onto the surface, including into the lakes. Evaporation or other processes could potentially induce precipitation of these organics, forming ‘bathtub rings’ similar to those observed by Cassini around some of the Northern lakes. These lake evaporites may play an important role in the surface chemistry of Titan.
We focus on benzene, which has been detected by Cassini in the Titan atmosphere and was tentatively identified on the surface by Huygens. Recent work in our laboratory shows that benzene has a very low solubility in liquid ethane (18.5 mg/L), meaning is a likely constituent of possible evaporites of Titan lakes.
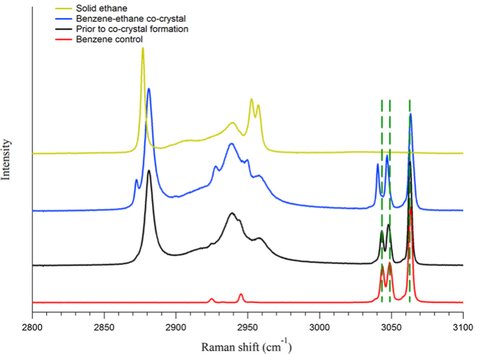
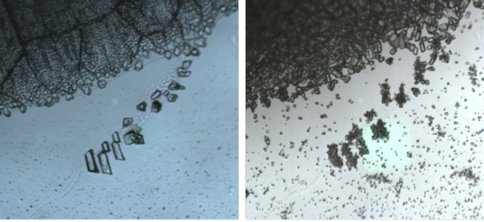
We performed a series of experiments in which solid benzene and liquid ethane were allowed to interact under Titan surface conditions (1 bar N2, 94 K). The interaction was studied using confocal Raman microscopy. On the basis of optical microscopy and the Raman spectra, we identified the formation of a distinctive co-crystalline structure. New features in the Raman spectrum at 2873 cm-1 and 1455 cm-1 were characteristic of the new structure (Fig 1). Recrystallization of the solid benzene was observed during the co-crystal formation (Fig 2).
Additional experiments were performed in order to characterize the temperature stability and kinetics of formation of the benzene:ethane co-crystal. We find that the co-crystal is stable up to ~150 K, and would form rapidly (<18 hours) at temperatures representative of Titan’s surface (94 K).
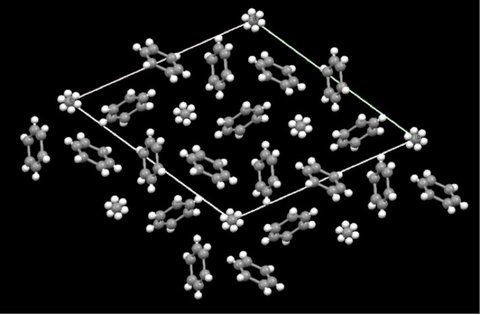
In cooperation with Dr. Helen Maynard-Casely, we were awarded beam time at the Australian Synchrotron (Melbourne, Australia) in order to study the crystal structure of the benzene:ethane co-crystal on the powder diffraction (PD) beamline. The PD beamline at the Australian synchrotron is ideally suited for these experiments, as facilities for cooling of the sample to cryogenic temperatures and the ability to condense gasses onto the sample are already in place. These experiments were highly successful, and the we were able to obtain a powder diffraction patter of the co-crystal and solve the structure (Fig. 3.) A paper describing these results is in progress.
Our work suggests that the benzene:ethane co-crystal may be the dominant form of benzene on titan wherever crystalline benzene has been in contact with liquid ethane, as in evaporite basins around ethane/methane lakes and seas. The benzene:ethane co-crystal represents a new class of materials for Titan’s surface, analogous to hydrated minerals on Earth. This new structure may also influence evaporite characteristics such as particle size, dissolution rate, and infrared spectral properties. Future work will involve searching for new organic co-crystal structures and their characterization.
Publications
-
Bennett, C. J., Pirim, C., & Orlando, T. M. (2013). Space-Weathering of Solar System Bodies: A Laboratory Perspective. Chem. Rev., 113(12), 9086–9150. doi:10.1021/cr400153k
-
Cable, M. L., Hörst, S. M., He, C., Stockton, A. M., Mora, M. F., Tolbert, M. A., … Willis, P. A. (2014). Identification of primary amines in Titan tholins using microchip nonaqueous capillary electrophoresis. Earth and Planetary Science Letters, 403, 99–107. doi:10.1016/j.epsl.2014.06.028
-
Cable, M. L., Vu, T. H., Hodyss, R., Choukroun, M., Malaska, M. J., & Beauchamp, P. (2014). Experimental determination of the kinetics of formation of the benzene-ethane co-crystal and implications for Titan. Geophysical Research Letters, 41(15), 5396–5401. doi:10.1002/2014gl060531
-
Dawley, M. M., Pirim, C., & Orlando, T. M. (2014). Radiation Processing of Formamide and Formamide:Water Ices on Silicate Grain Analogue. The Journal of Physical Chemistry A, 118(7), 1228–1236. doi:10.1021/jp4042815
-
Dawley, M. M., Pirim, C., & Orlando, T. M. (2014). Thermal Processing of Formamide Ices on Silicate Grain Analogue. The Journal of Physical Chemistry A, 118(7), 1220–1227. doi:10.1021/jp404026s
-
He, C., & Smith, M. A. (2014). A comprehensive NMR structural study of Titan aerosol analogs: Implications for Titan’s atmospheric chemistry. Icarus, 243, 31–38. doi:10.1016/j.icarus.2014.09.021
-
He, C., & Smith, M. A. (2014). Solubility and stability investigation of Titan aerosol analogs: New insight from NMR analysis. Icarus, 232, 54–59. doi:10.1016/j.icarus.2014.01.007
-
Jeilani, Y. A., Orlando, T. M., Pope, A., Pirim, C., & Nguyen, M. T. (2014). Prebiotic synthesis of triazines from urea: a theoretical study of free radical routes to melamine, ammeline, ammelide and cyanuric acid. RSC Adv., 4(61), 32375. doi:10.1039/c4ra03717k
-
Malaska, M. J., & Hodyss, R. (2014). Dissolution of benzene, naphthalene, and biphenyl in a simulated Titan lake. Icarus, 242, 74–81. doi:10.1016/j.icarus.2014.07.022
-
Vu, T. H., Cable, M. L., Choukroun, M., Hodyss, R., & Beauchamp, P. (2014). Formation of a New Benzene–Ethane Co-Crystalline Structure Under Cryogenic Conditions. The Journal of Physical Chemistry A, 118(23), 4087–4094. doi:10.1021/jp501698j
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Robert Hodyss
Project Investigator
Morgan Cable
Co-Investigator
Mathieu Choukroun
Co-Investigator
Tuan Vu
Co-Investigator
Patricia Beauchamp
Collaborator
Michael Malaska
Collaborator
-
RELATED OBJECTIVES:
Objective 1.1
Formation and evolution of habitable planets.
Objective 2.2
Outer Solar System exploration
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules