2014 Annual Science Report
 NASA Jet Propulsion Laboratory - Icy Worlds
Reporting | SEP 2013 – DEC 2014
NASA Jet Propulsion Laboratory - Icy Worlds
Reporting | SEP 2013 – DEC 2014
Astrobiology of Icy Worlds
Project Summary
Our goal in the Astrobiology of the Icy Worlds Investigation is to advance our understanding of the role of ice in the broad context of astrobiology through a combined laboratory, numerical, analytical, and field investigations. Icy Worlds team pursues this goal through four major investigations namely, the habitability, survivability, and detectability of life of icy worlds coupled with “Path to Flight” Technology demonstrations. A search for life linked to the search for water should naturally “follow the ice”. Can life emerge and thrive in a cold, lightless world beneath hundreds of kilometers of ice? And if so, do the icy shells hold clues to life in the subsurface? These questions are the primary motivation of our science investigations
Project Progress
Our goal in the Astrobiology of the Icy Worlds Investigation is to advance our understanding of the role of ice in the broad context of astrobiology through a combined laboratory, numerical, analytical, and field investigations. Icy Worlds team will pursue this goal through four major themes namely, the habitability, survivability, and detectability of life of icy worlds coupled with “*Path to Flight*” Technology demonstrations as briefly summarized below.
* Habitability of Icy Worlds investigates the habitability of liquid water environments in icy worlds, with a focus on what processes may give rise to life, what processes may sustain life, and what processes may deliver that life to the surface.
* Survivability of Icy Worlds investigates the survivability of biological compounds under simulated icy world surface conditions, and compare the degradation products to abiotically synthesized compounds resulting from the radiation chemistry on icy worlds.
* Detectability of Icy Worlds investigates the detectability of life and biological materials on the surface of icy worlds, with a focus on spectroscopic techniques, and on spectral bands that are not in some way connected to photosynthesis.
* Our technology investigation, a Path to Flight for astrobiology, utilizes instrumentation built with non-NAI funding to carry out the science investigations discussed above. The search for life requires instruments and techniques that can detect biosignatures from orbit and in-situ under harsh conditions. Advancing this capacity is the focus of our Technology Investigation.
In this reporting period, we continued to work on Habitability of Icy Worlds. As a part of Habitability Investigation, we investigated conditions relevant to sea floor processes that might be conducive to originating and supporting life in icy world interiors. To enable prediction testing by pursuing related questions, we will define biomarker production and look for ground-truth in analog terrestrial systems.
Our first task was involved in hydrothermal reactor experiments for origin of life. We continued to test the hypothesis that life can originate through a seafloor process that occurs at temperatures lower than those occurring in hydrothermal systems at mid-ocean ridges. Our objective was to analyze hydrothermal reactor effluents for methane by tunable diode laser spectroscopy (TLS) calibrated and set for both 12CH4 and 13CH4 detection. Investigators who contributed to this task are Michael Russell (lead), Isik Kanik, Steve Vance, Gordon Love, Max Coleman. Members: Laurie Barge, John Baross, Lance Christensen, Richard Kidd, Randall Mielke, and Lauren Spencer-White.
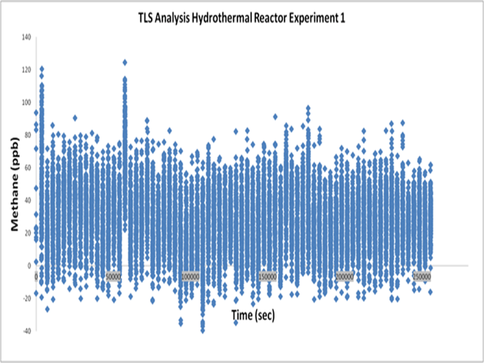
Although we had previously produced methane in our serpentinizing experiments involving komatiite and pyrrhotite/pentlandite, its source, whether scrubbed from source rock or reduced from the CO2 feed, was unclear. Therefore we undertook the same experiment in the absence of CO2 and, in the event, obtained up to ~60 ppb which we reasoned was derived from organic molecules and/or methane sequestered in the natural pyrrhotite.(Fig. 1).
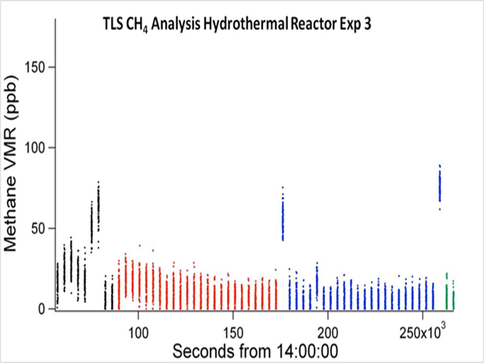
In experiments conducted with 99.99% enriched 13CO2, measured 13C-enriched methane present was below the detection limit of the instrument (LOD = 1.8 ppb) (Fig. 2). We also failed to generate the thermodynamically challenging formaldehyde (HCHO).
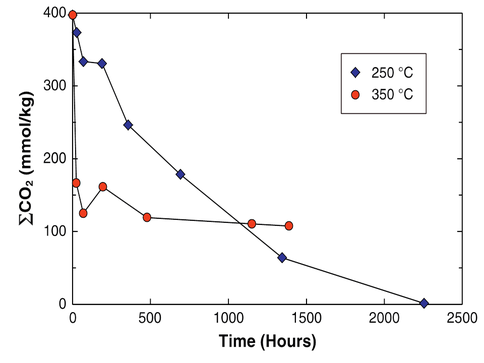
Given this result we reacted komatiitic and basaltic rocks with CO2 to understand its fate at high-pressures and temperatures. At up to 250 °C and 500 bars, total carbonic acid concentration (ΣCO2) reduced from its initial 400 mmol/kg to near zero, while at higher temperatures just over 100 mM/kg remained in solution (Shibuya et al., 2013a) (Fig. 3).
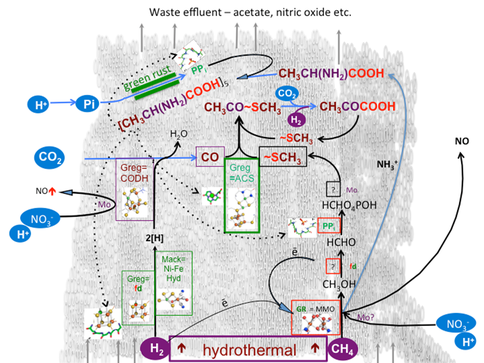
Rather than reducing the CO2 to methane we conclude that CO2 in the ancient oceans was mainly lost to carbonation of the crust (Shibuya et al., 2013a and b). To explain the first steps of metabolism involved disequilibria between the high potential electron acceptor nitrate and the fuels H2 and CH4 exhaling from alkaline springs (Figure 4). We conclude that the acetyl co-A pathway was but driven from both ends, i.e., through the reduction of CO2 to formate and CO as we have partially demonstrated (Figure 4), and the concomitant oxidation of methane to methanol, formaldehyde, methylene and a methyl group, and its subsequent condensation to activated acetate in what we term “the denitrifying methanotrophic acetogenic pathway.”
This opens up the possibility of the first emergence of a ligand-accelerated autocatalytic cycle (Figure 4). Short peptides condensed from simple amino acids such as alanine would ligate, nest, and so protect the inorganic clusters, improving their substrate affinities and catalytic activities as well as, in the case of the iron sulfides, stabilizing the reduced rather than oxidized forms, so appreciably lowering their reduction potential. Another benefit would be to prevent passivation of Ni-Fe sulfides and thereby improve redox reversibility of any hydrogenase precursor. Such positive feedbacks would quicken, and help direct, these advantageous protobiotic reactions even before the emergence of a nucleic acid guidance system and the ensuing RNA world would effectively lock these steps into biochemistry. Abstracting and examining the enzymes that currently facilitate these mostly steep endergonic barriers may indicate mineral candidates that may, as conformational protoenzymes as well as simple catalysts, have first managed the endergonic/exergonic couplings required to see to life’s onset. Because true catalysts have no influence on the direction of a reaction, various disequilibria are always required to drive catalytic pathways and cycles, including the ambient proton gradient – a precursor of the proton motive force .
One of the tasks in this investigation was to obtain equations of state (thermodynamic properties) for aqueous ammonia under deep ocean pressures and temperatures, incorporate new data and existing MgSO4 data into thermodynamic databases and investigate additional solutions as time permits.
A major goal of the interiors project is to provide tools for computing energies of water-rock and metabolic reactions that are needed to truly assess habitability in deep oceans.
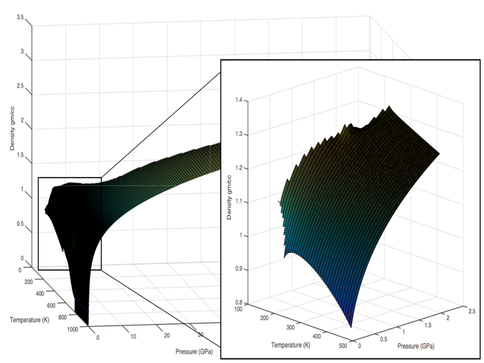
Co-I J. Michael Brown has developed new fitting methods for icy world equations of state that extend thermodynamic properties of water to multiple GPa pressures applicable to the deepest oceans. The new methods use grid regularization and smoothing splines, standard tools in geophysical inverse theory, to create smooth surfaces in sound speed, density, and heat capacity that fit available data to within the errors of measurement. Gibbs free energy surfaces (the pressure integral of specific volume and temperature integral of heat capacity) provide compact and highly accurate representations of the thermodynamics for icy world ocean solutions.
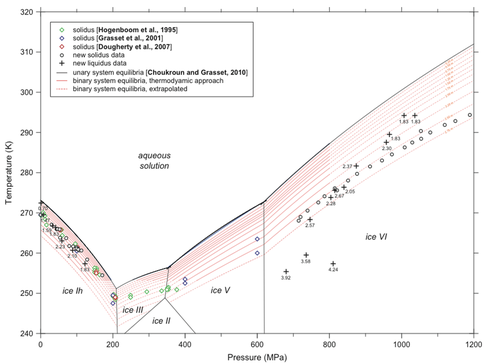
The new thermodynamic tools are revolutionary for their broad applicability in pressure and temperature, but their real power comes from application to multi-component and multiphase solutions that define planetary oceans covered with ice. Co-I Vance has developed Pitzer thermodynamic parameters for aqueous magnesium sulfate (Phutela and Pitzer 1986) that allow the team to express their thermodynamic equation of state for MgSO4 (Vance and Brown 2013) in terms of the new Gibbs energy framework and in a way that is referenced to formulations for other aqueous systems. This innovation clears the path to both computing melting and freezing phase equilibria and salt precipitation for the MgSO4 system.
Postdoc Olivier Bollengier has been working with the Icy Worlds team, leveraging Outer Planets research funding to advance relevant goals of the UW mineral physics lab. Dr. Bollengier applied the new thermodynamic framework for aqueous MgSO4 to ice melting equilibrium measurements made by him and others. The resulting equation of state can be used to reexamine models for interior structure of Ganymede (Vance et al. 2014), and to extend that work to higher ocean salinities. Future efforts will apply the interior structure model to other icy worlds, Titan, Callisto, and Triton, and to watery exoplanets that will be revealed in increasing detail by the upcoming Transiting Exoplanet Synoptic Survey (TESS; Ricker et al. 2014).
Efforts underway are creating similar equations of state for aqueous ammonia based on experimental results by Vance and other members of the mineral physics laboratory (Vance et al. in prep., Abramson 2008). The Pitzer framework allows for development of multi-component equations of state for MgSO4 and ammonia.
The team plans to focus in the near term on publishing the respective equations of state for water, MgSO4 (with a focus on ice-water equilibria), and NH3, including initial applications of these results to understanding planetary interiors. Preliminary results for Titan take into account the recent insight that interior structure measurements based on gravitational moments of inertia for slowly rotating worlds may be in error by up to 10%. The result is that Titan and Callisto may be more strongly differentiated. In that case, a 400 km deep ocean in either world can easily have a seafloor unobstructed by high-pressure ices.
As a part of Icy Worlds Survivability investigation, investigators Paul Johnson, Robert Hodyss, Aaron Noell and Adrian Ponce, along with NPP Postdoc Dr. Jeff Hein completed and published an investigation into the spectral properties of bacterial spores in cryogenic ices as well as their viability under solar radiation. Bacterial spores are one of the toughest and most durable forms of life on earth. Can bacterial spores embedded in near surface Europan ice survive the Jovian radiation environment? Can either the spores or their organic radiolysis products be detected spectroscopically through remote sensing?
Bacillus subtillus was chosen for not only its ability to readily generate spores, but its well-established use as a model species. It is a useful tracker of UV irradiation effects because of its high resilience to a variety of extreme conditions. As such it is an important indicator species for forward contamination in the context of planetary protection. Purified spores were deposited onto silicon-oxide coated aluminum mirrors in both a monolayer, and multilayer form. The multilayer form, which generated a substantially increased FT-IR signal, was initially used to more clearly identify the known absorptive features of spores at standard temperature. The monolayer was then substituted in to make the radiation effects more reproducible. The monolayer spores were stamped onto half of the mirror (the remaining half used as a background blank), whereas the multilayer took the form of a noticeable spore spot on the mirror ~2mm in diameter. Once prepared, the mirrors were mounted on a cryostat and transferred to a vacuum apparatus for UV irradiation and FT-IR analysis. For viability studies the spores were deposited on nickel plated stainless steel sample tabs. Here monolayers were used to allow for more accurate post-UV viability testing ensuring that all spores received an equal UV dosage and were not protecting one another.
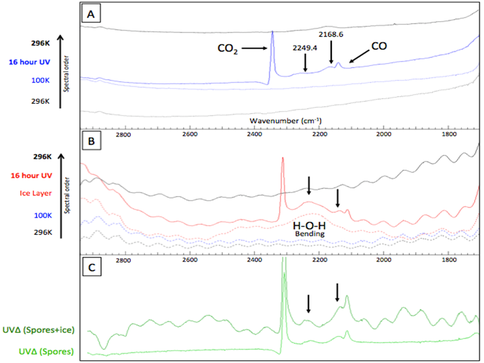
Briefly, they found that B. subtilis spores generate low molecular weight photoproducts when irradiated with UV at low temperature and pressures (100K and 10-9 Torr). After irradiation, absorption bands were observed at 2249.4 and 2168.7 cm-1, which is within the general region of nitrile containing groups, though no positive identification has yet been achieved (Figure 7). Looking at the evolved gases with a mass spectrometer while heating the samples showed that the associated irradiation-induced photoproducts generate secondary products upon the ionization with masses of 14.2 and 40.2 amu. The addition of a thin (0.25μm) water-ice layer on top of the spores did not appear to alter or interfere with the UV induced photochemistry and accompanying products.
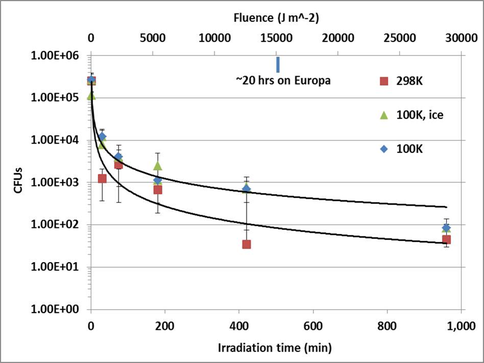
Results of the viability work are seen in figure 8. We find increased viability of spores irradiated at 100K vs room temperature. Future research will greatly expand the scope of the project to include (i) spore survivability as a function of ice thickness, (iii) spore spectral signature alteration as a function of ice thickness, and (iv) the identification of the aforementioned photoproducts with accompanying chemistry.
As far as the Detectability of Icy Worlds investigation is concerned, Co-I Dalton and co-workers investigated the surface chemistry of Mars, an icy body, which was partially supported by NAI. In their paper, Dalton and co-workers reported Visible and near-infrared wavelength (VNIR, lambda = 0.35-5 mu m) laboratory diffuse reflectance spectra and corresponding optical functions (real and imaginary refractive indices) for several iron sulfates (natural K- and Na-jarosite, szomolnokite, rhomboclase). On Mars, jarosite has been identified in Meridiani Planum, Mawrth Vallis, Melas Chasma, and Eridania Basin; szomolnokite has been found as distinct layers at Columbus Crater and as outcrops at Juventae Chasma, and rhomboclase has been identified at Gusev Crater. Constraining the mineralogy and chemistry (Fe- vs. Mg-rich) of the sulfates on Mars may contribute to our understanding of the environmental and aqueous conditions present on Mars during their formation. These data will help to constrain the mineralogy, abundance, and distribution of sulfates on the martian surface, which will lead to improvements in understanding the pressure, temperature, and humidity conditions and how active frost, groundwater, and atmospheric processes once were on Mars.
Another study relevant to the Detectability of Icy Worlds investigation was conducted by involvement of Co-I’s Kevin Hand and Brad Dalton from Icy Worlds team regarding weathering effect of surfaces Jovian and Saturnian satellites. The satellites of Jupiter and Saturn can be weathered by a number of different agents, including micrometeroids, plasma, electrons and photons. Understanding the effects these agents have on surface materials is important for separating endogenic and exogenic features, as well as for tracing specific molecules back to their source.
They have modeled an electron precipitation pattern expected on Mimas, Tethys, and Dione, using two different approaches. In the first approach, they adapt a previously developed model to compute an integrated energy flux into the surfaces of Mimas, Tethys, and Dione. This is a guiding-center, bounce averaged model. In the second approach, they track individual particles in an electromagnetic field for an inert or slightly magnetized satellite. This second approach allows them to include the effects of electron pitch angle and gyrophase on the weathering pattern. Both methods converge on an enhanced dose pattern on each satellite’s leading hemisphere that is lens-shaped. They also present mission-averaged electron energy spectra obtained near these satellites by Cassini’s Magnetosphere Imaging Instrument (MIMI). These data are interpreted using their current understanding of both the environment and the instrument’s response. Fits to the data are integrated to find an energy flux into each satellite’s surface, as a function of longitude and latitude. Using positions on the moon accessible to energetic electrons from the modeling and the integrated energy flux based on data, they find lens patterns that fall off with increasing moon latitude. The predicted patterns are qualitatively consistent with some but not all of the optical observations made of these hemispheres.
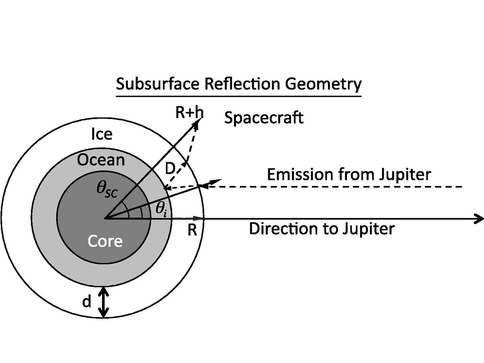
As far as the Field Instrumentation and Path to Flight investigation’s is concerned, Co-I Vance worked closely with a team at JPL to develop a new concept for probing the subsurface of Europa’s ice shell using Jupiter’s decametric emission (Romero-Wolf et al. 2014). This investigation employed an interferometric reflectometer method for passive detection of subsurface oceans and liquid water in jovian icy moons using Jupiter’s decametric radio emission (DAM) (Figure 9). The DAM flux density exceeds 3000 times the galactic background in the neighborhood of the jovian icy moons, providing a signal that could be used for passive radio sounding. An instrument located between the icy moon and Jupiter could sample the DAM emission along with its echoes reflected in the ice layer of the target moon. Cross-correlating the direct emission with the echoes would provide a measurement of the ice shell thickness along with its dielectric properties. The interferometric reflectometer provides a simple solution to sub-jovian radio sounding of ice shells that is complementary to ice penetrating radar measurements better suited to measurements in the anti-jovian hemisphere that shadows Jupiter’s strong decametric emission. The passive nature of this technique also serves as risk reduction in case of radar transmitter failure. The interferometric reflectometer could operate with electrically short antennas, thus extending ice depth measurements to lower frequencies, and potentially providing a deeper view into the ice shells of jovian moons.
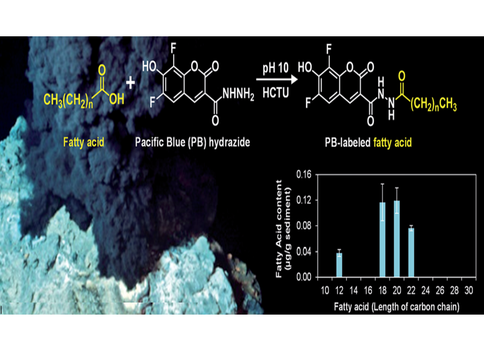
Co-I Hand has involved in demonstration of nonaqueous labeling and separation of the full range of short to long saturated fatty acids (C2 to C30) for the first time on a microfluidic device. A new fluorescent dye, Pacific Blue hydrazide, labels the carboxylic acid in a two-step, one-pot reaction to enable detection via laser-induced fluorescence at 405 nm excitation. Limits of detection for C10 to C30 acids range from 0.9 to 5.7 mu M. Fatty acids were successfully quantified in a sediment sample from the 'Snake Pit’ hydrothermal system of the Mid-Atlantic Ridge, demonstrating the potential of this method to help characterize microbial communities through targeted biomarker analysis. Such a technique could also be utilized to differentiate between abiotic and biotic compounds in the search for life beyond Earth.
As a part of Path to Flight, we continued to make great progress in “LIFE” mission concept (see Tsou et al., 2012) investigation for Enceladus, a low-cost sample return mission to a body with high astrobiological potential.
References:
Abramson, E. H. (2008). Speeds of sound in fluid ammonia to 3.8 GPa and 680 K. Journal of Chemical & Engineering Data, 53(8):1986–1987.
Ducluzeau, A. L., Schoepp-Cothenet, B., van Lis, R., Baymann, F., Russell, M. J., & Nitschke, W. (2014a). The evolution of respiratory O2/NO reductases: an out-of-the-phylogenetic-box perspective. J. R. Soc. Interface, 11, 20140196; doi: 10.1098/rsif.2014.0196
Ducluzeau, A-L., Schoepp-Cothenet, B., Baymann, F., Russell, M.J. & Nitschke, W. (2014b) Free energy conversion in the LUCA: Quo vadis? Biochim. Biophys. Acta, Bioenergetics, http://dx.doi.org/ 10.1016/j.bbabio.2013.12.005
Phutela, R. C. and Pitzer, K. S. (1986). Heat capacity and other thermodynamic properties of aqueous magnesium sulfate to 473 K. Journal of Physical Chemistry, 90:895–901.
Ricker, G. R., Winn, J. N., Vanderspek, R., Latham, D. W., Bakos, G. A ́., Bean, J. L., Berta-Thompson, Z. K., Brown, T. M., Buchhave, L., Butler, N. R., et al. (2014). Transiting Exoplanet Survey Satellite (TESS). In SPIE Astronomical Telescopes+ Instrumentation, pages 914320– 914320. International Society for Optics and Photonics.
Romero-Wolf, A., Vance, S., Maiwald, F., Heggy, E., Ries, P., and Liewer, K. (2014). A passive probe for subsurface oceans and liquid water in jupiter’s icy moons. Icarus, (to appear).
Russell, M.J., Barge, L.M., Bhartia, R., Bocanegra, D., Bracher, P.J., Branscomb, E., Kidd, R., McGlynn, S.E., Meier, D.H., Nitschke, W., Shibuya, T., Vance, S., White, L., & Kanik, I. (2014) The drive to life on wet and icy worlds. Astrobiology 14, 308-343.
Shibuya, T., Tahata, M., Ueno, Y., Komiya, T., Takai, K., Yoshida, N., Maruyama, S., & Russell, M. J. (2013b). Decrease of seawater CO2 concentration in the Late Archean: An implication from 2.6 Ga seafloor hydrothermal alteration. Precambrian Research, 236, 59-64.
Shibuya, T., Yoshizaki, M., Masaki, Y., Suzuki, K., Takai, K., & Russell, M. J. (2013a). Reactions between basalt and CO2-rich seawater at 250 and 350° C, 500bars: Implications for the CO2 sequestration into the modern oceanic crust and the composition of hydrothermal vent fluid in the CO2-rich early ocean. Chemical Geology, 359, 1-9.
Tsou, P., Brownlee, D. E., McKay, C. P.; et al., LIFE: Life Investigation For Enceladus A Sample Return Mission Concept in Search for Evidence of Life , (2012) ASTROBIOLOGY, 12, Issue: 8 Pages: 730-742 DOI10.1089/ast.2011.0813
Vance, S. and Brown, J. (2013). Thermodynamic properties of aqueous MgSO4 to 800 MPa at temperatures from -20 to 100 oC and concentrations to 2.5 mol kg−1 from sound speeds, with applications to icy world oceans. Geochimica et Cosmochimica Acta, 110:176–189.
Vance, S., Bouffard, M., Choukroun, M., and Sotin, C. (2014). Ganymede’s internal structure including thermodynamics of magnesium sulfate oceans in contact with ice. Planetary And Space Science, 96:62–70.
Publications
-
Barge, L. M., Doloboff, I. J., Russell, M. J., VanderVelde, D., White, L. M., Stucky, G. D., … Kanik, I. (2014). Pyrophosphate synthesis in iron mineral films and membranes simulating prebiotic submarine hydrothermal precipitates. Geochimica et Cosmochimica Acta, 128, 1–12. doi:10.1016/j.gca.2013.12.006
-
Barge, L. M., Kee, T. P., Doloboff, I. J., Hampton, J. M. P., Ismail, M., Pourkashanian, M., … Kanik, I. (2014). The Fuel Cell Model of Abiogenesis: A New Approach to Origin-of-Life Simulations. Astrobiology, 14(3), 254–270. doi:10.1089/ast.2014.1140
-
Ducluzeau, A-L., Schoepp-Cothenet, B., Van Lis, R., Baymann, F., Russell, M. J., & Nitschke, W. (2014). The evolution of respiratory O2/NO reductases: an out-of-the-phylogenetic-box perspective. Journal of The Royal Society Interface, 11(98), 20140196–20140196. doi:10.1098/rsif.2014.0196
-
McKay, C. P., Anbar, A. D., Porco, C., & Tsou, P. (2014). Follow the Plume: The Habitability of Enceladus. Astrobiology, 14(4), 352–355. doi:10.1089/ast.2014.1158
-
Noell, A. C., Ely, T., Bolser, D. K., Darrach, H., Hodyss, R., Johnson, P. V., … Ponce, A. (2015). Spectroscopy and Viability of Bacillus subtilis Spores after Ultraviolet Irradiation: Implications for the Detection of Potential Bacterial Life on Europa. Astrobiology, 15(1), 20–31. doi:10.1089/ast.2014.1169
-
Paranicas, C., Roussos, E., Decker, R. B., Johnson, R. E., Hendrix, A. R., Schenk, P., … Mitchell, D. G. (2014). The lens feature on the inner saturnian satellites. Icarus, 234, 155–161. doi:10.1016/j.icarus.2014.02.026
-
Pitman, K. M., Noe Dobrea, E. Z., Jamieson, C. S., Dalton, J. B., Abbey, W. J., & Joseph, E. C. S. (2014). Reflectance spectroscopy and optical functions for hydrated Fe-sulfates. American Mineralogist, 99(8-9), 1593–1603. doi:10.2138/am.2014.4730
-
Ricker, G. R., Winn, J. N., Vanderspek, R., Latham, D. W., Bakos, G. Á., Bean, J. L., … Villasenor, J. (2014). Transiting Exoplanet Survey Satellite (TESS). Space Telescopes and Instrumentation 2014: Optical, Infrared, and Millimeter Wave. doi:10.1117/12.2063489
-
Romero-Wolf, A., Vance, S., Maiwald, F., Heggy, E., Ries, P., & Liewer, K. (2015). A passive probe for subsurface oceans and liquid water in Jupiter’s icy moons. Icarus, 248, 463–477. doi:10.1016/j.icarus.2014.10.043
-
Russell, M. J., Barge, L. M., Bhartia, R., Bocanegra, D., Bracher, P. J., Branscomb, E., … Kanik, I. (2014). The Drive to Life on Wet and Icy Worlds. Astrobiology, 14(4), 308–343. doi:10.1089/ast.2013.1110
-
Shibuya, T., Tahata, M., Ueno, Y., Komiya, T., Takai, K., Yoshida, N., … Russell, M. J. (2013). Decrease of seawater CO2 concentration in the Late Archean: An implication from 2.6Ga seafloor hydrothermal alteration. Precambrian Research, 236, 59–64. doi:10.1016/j.precamres.2013.07.010
-
Vance, S., Bouffard, M., Choukroun, M., & Sotin, C. (2014). Ganymede׳s internal structure including thermodynamics of magnesium sulfate oceans in contact with ice. Planetary and Space Science, 96, 62–70. doi:10.1016/j.pss.2014.03.011
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Isik Kanik
Project Investigator
Brad Dalton
Co-Investigator
Jason Goodman
Co-Investigator
Kevin Hand
Co-Investigator
Paul Johnson
Co-Investigator
Adrian Ponce
Co-Investigator
Michael Russell
Co-Investigator
Christophe Sotin
Co-Investigator
Steven Vance
Co-Investigator
Laura Barge
Collaborator
Lance Christensen
Collaborator
Robert Hodyss
Collaborator
Richard Kidd
Collaborator
Christopher McKay
Collaborator
Lauren Spencer-White
Collaborator
Peter Tsou
Collaborator
-
RELATED OBJECTIVES:
Objective 1.1
Formation and evolution of habitable planets.
Objective 2.1
Mars exploration.
Objective 2.2
Outer Solar System exploration
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules
Objective 4.1
Earth's early biosphere.
Objective 5.1
Environment-dependent, molecular evolution in microorganisms
Objective 5.2
Co-evolution of microbial communities
Objective 5.3
Biochemical adaptation to extreme environments
Objective 6.2
Adaptation and evolution of life beyond Earth
Objective 7.1
Biosignatures to be sought in Solar System materials