2014 Annual Science Report
 NASA Goddard Space Flight Center
Reporting | SEP 2013 – DEC 2014
NASA Goddard Space Flight Center
Reporting | SEP 2013 – DEC 2014
Fischer-Tropsch-Type Reactions in the Solar Nebula
Project Summary
Fischer-Tropsch-Type (FTT) reactions can form complex hydrocarbons via surface-mediated reactions using simple gases (CO, N2, and H2) on almost any grain surface and are currently being studied in relation to the early Solar Nebula. Several theories exist as to how hydrocarbons are formed in the early Solar System but the compelling nature of this type of reaction is that it is passive and generates a wide variety of complex hydrocarbons using commonly available components (gases/grains) without invoking a complex set of conditions for formation.
Project Progress
Fischer-Tropsch Type (FTT) Reactions in the Solar Nebula
Joseph Nuth, Natasha Johnson, and Frank Ferguson
Fischer-Tropsch-Type (FTT) reactions can form complex hydrocarbons via surface-mediated reactions using simple gases (CO, N2, and H2) on almost any grain surface and are currently being studied in relation to the early Solar Nebula. Several theories exist as to how hydrocarbons are formed in the early Solar System but the compelling nature of this type of reaction is that it is passive and generates a wide variety of complex hydrocarbons using commonly available components (gases/grains) without invoking a complex set of conditions for formation. This method for generating hydrocarbons is important because it provides insight or potential as to how comets, meteorites, and the early Earth may have obtained their first hydrocarbon inventory. In this reporting year, we expanded our earlier FTT experiments into several related areas of interest, including the formation of amino acids, trapping of noble gases by newly formed complexes, and the sequestration of solid carbon.
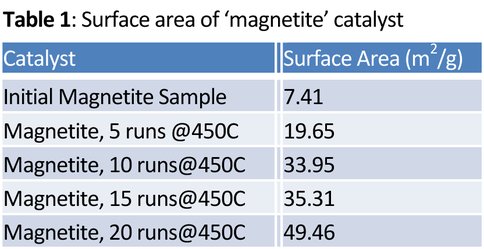
We ran numerous simultaneous experiments using our multiple (10-set) FTT system to investigate reaction efficiencies and rates for iron, magnetite (Fe3O4), and hematite (Fe2O3). The hematite was quickly discovered to reduce to magnetite under the predominantly H2 gas mixture and was dropped from the current line of investigation. The experiments are designed to quantitatively measure the solid/gas product ratio as a function of catalyst, time, temperature, and run number. For example, Table 1 summarizes typical surface area measurements from successive magnetite experiments and clearly demonstrates that significant carbon is deposited on the initial substrate. This surface area change (m2/g) is due to the deposition of complex hydrocarbons during each run cycle.
We also continued long-term low-temperature (200-300°C) FTT experiments on synthesized metal-silicate dust that included noble gases in the mix; two such experiments have been running continuously since early 2013. These low temperatures are thought to be favorable for trapping noble gases within the deposited organic coating. There appears to be some slow hydrocarbon production using Fe-silicate dust and if there is sufficient organic deposition, then the samples will be sent out for noble gas analyses in 2015.
During the summer of 2014, we were joined by Alicia Behhidjeb-Carayon, a Masters intern from the International Space University. Alicia systematically explored magnetite as an FTT catalyst through temperature and reaction cycles, specifically targeting the deposition of carbon. The year 2014 also saw the beginnings of collaboration with Darren Locke at Johnson Space Center who tackled the investigation of resultant carbon coating by utilizing techniques typically employed by the coal industry. By using the combination of pyrolysis GCMS with high resolution SEM imagery, we gained insights into both the morphology of the coating (relates directly to the change in surface area) and the possible sequence of solid hydrocarbon formation/deposition. Our work with the Astrobiology Analytical Lab also continues as we lay the groundwork for studies of amino acid formation in FTT reactions.
Publications
-
Nuth, I. J., Johnson, N., & Hill, H. (2014). CO Self-Shielding as a Mechanism to Make 16O-Enriched Solids in the Solar Nebula. Challenges, 5(1), 152–158. doi:10.3390/challe5010152
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Joseph Nuth
Project Investigator
Frank Ferguson
Co-Investigator
Natasha Johnson
Co-Investigator
Aaron Burton
Collaborator
Jason Dworkin
Collaborator
Hany Sobhi
Collaborator
-
RELATED OBJECTIVES:
Objective 1.1
Formation and evolution of habitable planets.
Objective 2.2
Outer Solar System exploration
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules