2014 Annual Science Report
 NASA Ames Research Center
Reporting | SEP 2013 – DEC 2014
NASA Ames Research Center
Reporting | SEP 2013 – DEC 2014
Cosmic Distribution of Chemical Complexity
Project Summary
This project explores the connections between chemistry in space and the origin of life. It is comprised of three tightly interwoven tasks. We track the formation and evolution of chemical complexity in space starting with simple carbon-rich molecules such as formaldehyde and acetylene. We then move on to more complex species including amino acids, nucleic acids and polycyclic aromatic hydrocarbons. The work focuses on carbon-rich species that are interesting from a biogenic perspective and on understanding their possible roles in the origin of life on habitable worlds. We do this by measuring the spectra and chemistry of analog materials in the laboratory, by remote sensing with small spacecraft, and by analysis of extraterrestrial samples returned by spacecraft or that fall to Earth as meteorites. We then use these results to interpret astronomical observations made with ground-based and orbiting telescopes.
Project Progress
Over the course of the past year the Astrochemistry Laboratory has continued to study the chemistry that happens in space and the possibility that the organic products of this chemistry may be incorporated into forming planets and play a role in any subsequent origin of life. This work has largely centered around the study of (i) radiation-induced chemistry that happens in astrophysical mixed-molecular ices of the types found in interstellar clouds and protostellar disks, and (ii) polycyclic aromatic hydrocarbons (PAHs), which are one of the main forms of molecular carbon in the universe.
Over the past year, the study of ice irradiation chemistry has focused on two, largely unrelated, issues. First, we have carried out numerous simulations to study the radiation-induced chemistry that can occur in Pluto ice analogs when they are dosed with UV photons or electrons. Ices on Pluto are dominated by N2, but also contain small amounts of CO, CO2, and CH4. This work has demonstrated that irradiation of these ices leads to the formation of more complex organics that contain carboxylic acids, nitriles, and urea (Materese et al. 2014). These results are of interest in their own right, but are also of interest because the infrared spectra obtained form these samples will be used to help interpret data returned from Pluto by the New Horizons spacecraft when it flies by Pluto later this year.
The other main task associated with ices has been the completion of our work on studying the formation of the pyrimidine-based nucleobases and other heterocyclic molecules. We have now demonstrated that all three of the pyrimidine-based nucleobases – uracil, cytosine, and thymine – can be made when pyrimidine is irradiation in mixed-molecular ices.
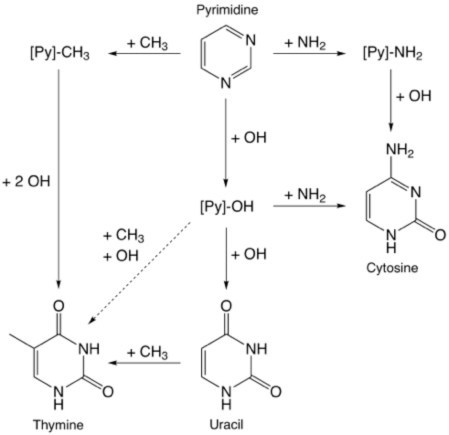
Interestingly, uracil and cytosine are fairly easy to make, while the efficiency of production of thymine is much smaller. It has been posited that the origin and evolution of life may have passed through an RNA World stage that preceded the use of DNA. Our results may provide an explanation of why this might have occurred – uracil may have been much more abundantly available in the early stages of Earth’s history than thymine. In related work, we have also recently shown that ice photochemistry can result in the O or N substitution of the skeletal C atoms in PAHs, i.e., PAHs irradiated in ices can be converted into O- and N-heterocycles (Materese et al. 2015). This process may be responsible for making molecules like pyrimidine from which the nucleobases are formed.
This past year’s work on PAHs has focused on two areas, (i) the interactions of these molecules with water ice as well as their photo-induced chemical reactions and (ii) interpreting Spitzer observations using the PAH database. Regarding (i), we recently published papers describing the impact PAH:water ice concentrations have on the resulting photo-chemistry (Cuylle et al. 2014, Cook et al. 2014). We have also been exploring the interaction of aromatic molecules, such as PAHs, with mineral substrates as well as any UV induced catalytic effects. The initial portion of this work was published in Langmuir ( Elesaessar et al. 2014).
This article was awarded the cover of the prestigious journal and was selected as the ACS (American Chemical Society) Editor’s Choice article for the month in which it was published. Concerning (ii), we released a significantly updated version of the NASA Ames PAH IR Spectroscopic Database that includes very large PAHs and the ability for users to import their astronomical spectra and perform least squares fitting analysis (Boersma et al. 2014a), and quantified the traditional qualitative proxy used to evaluate PAH charge distribution in the various astronomical emitting regions (Boersma et al. 2014b).
Publications
-
Cook, A. M., Mattioda, A. L., Quinn, R. C., Ricco, A. J., Ehrenfreund, P., Bramall, N. E., … Walker, R. (2014). SEVO ON THE GROUND: DESIGN OF A LABORATORY SOLAR SIMULATION IN SUPPORT OF THE O/OREOS MISSION. The Astrophysical Journal Supplement Series, 210(2), 15. doi:10.1088/0067-0049/210/2/15
-
Cook, A. M., Mattioda, A. L., Ricco, A. J., Quinn, R. C., Elsaesser, A., Ehrenfreund, P., … Hoffmann, S. V. (2014). The Organism/Organic Exposure to Orbital Stresses (O/OREOS) Satellite: Radiation Exposure in Low-Earth Orbit and Supporting Laboratory Studies of Iron Tetraphenylporphyrin Chloride. Astrobiology, 14(2), 87–101. doi:10.1089/ast.2013.0998
-
Cook, A. M., Ricca, A., Mattioda, A. L., Bouwman, J., Roser, J., Linnartz, H., … Allamandola, L. J. (2015). PHOTOCHEMISTRY OF POLYCYCLIC AROMATIC HYDROCARBONS IN COSMIC WATER ICE: THE ROLE OF PAH IONIZATION AND CONCENTRATION. The Astrophysical Journal, 799(1), 14. doi:10.1088/0004-637x/799/1/14
-
Elsaesser, A., Quinn, R. C., Ehrenfreund, P., Mattioda, A. L., Ricco, A. J., Alonzo, J., … Santos, O. (2014). Organics Exposure in Orbit (OREOcube): A Next-Generation Space Exposure Platform. Langmuir, 30(44), 13217–13227. doi:10.1021/la501203g
-
Materese, C. K., Cruikshank, D. P., Sandford, S. A., Imanaka, H., Nuevo, M., & White, D. W. (2014). ICE CHEMISTRY ON OUTER SOLAR SYSTEM BODIES: CARBOXYLIC ACIDS, NITRILES, AND UREA DETECTED IN REFRACTORY RESIDUES PRODUCED FROM THE UV PHOTOLYSIS OF N 2 :CH 4 :CO-CONTAINING ICES. The Astrophysical Journal, 788(2), 111. doi:10.1088/0004-637x/788/2/111
-
Mattioda, A. L., Bauschlicher, C. W., Bregman, J. D., Hudgins, D. M., Allamandola, L. J., & Ricca, A. (2014). Infrared vibrational and electronic transitions in the dibenzopolyacene family. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 130, 639–652. doi:10.1016/j.saa.2014.04.017
-
Rosenberg, M. J. F., Berné, O., & Boersma, C. (2014). Random mixtures of polycyclic aromatic hydrocarbon spectra match interstellar infrared emission. A&A, 566, L4. doi:10.1051/0004-6361/201423953
-
Sandford, S. A., Bera, P. P., Lee, T. J., Materese, C. K., & Nuevo, M. (2014). Photosynthesis and Photo-Stability of Nucleic Acids in Prebiotic Extraterrestrial Environments. Topics in Current Chemistry, None, 123–164. doi:10.1007/128_2013_499
-
Yabuta, H., Uesugi, M., Naraoka, H., Ito, M., Kilcoyne, A. L. D., Sandford, S. A., … Abe, M. (2014). X-ray absorption near edge structure spectroscopic study of Hayabusa category 3 carbonaceous particles. Earth Planet Sp, 66(1), None. doi:10.1186/s40623-014-0156-0
-
Yada, T., Fujimura, A., Abe, M., Nakamura, T., Noguchi, T., Okazaki, R., … Kawaguchi, J. (2013). Hayabusa-returned sample curation in the Planetary Material Sample Curation Facility of JAXA. Meteoritics & Planetary Science, 49(2), 135–153. doi:10.1111/maps.12027
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Louis Allamandola
Project Investigator
Christiaan Boersma
Co-Investigator
Andrew Mattioda
Co-Investigator
Michel Nuevo
Co-Investigator
Scott Sandford
Co-Investigator
Amanda Cook
Collaborator
-
RELATED OBJECTIVES:
Objective 2.2
Outer Solar System exploration
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules
