2013 Annual Science Report
 University of Wisconsin
Reporting | SEP 2012 – AUG 2013
University of Wisconsin
Reporting | SEP 2012 – AUG 2013
Project 3A: Banded Iron Formation Deposition Across the Archean-Proterozoic Boundary
Project Summary
Prior to widespread oxygenic photosynthesis, reduced iron, Fe(II), was the dominant form of soluble iron in surface environments on the early Earth, and likely Mars. On Earth, extensive iron deposits, Banded Iron Formations (BIFs), which currently supply the majority of the iron used in our society, largely formed prior to the Great Oxidation Event of ~2.4 Ga age, and yet contain substantial quantities of oxidized iron, Fe(III). The pathways by which these different oxidation states arose remains unclear. In addition, the chemical and isotopic compositions of BIFs have been used as proxies for ancient seawater or paleoenvironments. In competition with this proposal, however, has been use of BIFs as a tracer of microbial iron cycling. To test the use of BIFs as ambient paleoenvironmental proxies or proxies of microbial process, BIFs from South Africa and Australia were examined from the micron scale to the 100’s of meter scales. We find that BIFs tend to record specific pathways of oxidation of Fe(II), as well as reduction of Fe(III), and extensive post-depositional changes, and it may be quite difficult to infer ambient paleoenvironmental conditions form such deposits.
Project Progress
The abundance of Precambrian Banded Iron Formations (BIFs) comprises one of the major lines of evidence for an Fe(II)-rich ocean in the Archean and Paleoproterozoic. In addition, Fe(II)-bearing minerals in IFs, including siderite and magnetite, have been used to constrain ambient CO2 contents in the ancient Earth. In contrast, mineralogical, chemical, and isotopic data have documented the extensive post-formation changes that occur during early diagenesis through metamorphism in BIFs, which questions the use of BIFs as a paleoenvironmental proxy. It remains unclear what pathways produced the mixed oxidation state of BIFs, where both Fe(II) and Fe(III) minerals are abundant. Such insights may bear on understanding the mixed oxidation states of Fe on Mars, as recently highlight by analyses by the rover Curiosity and the Mars Science Lab mission.
To better understand the pathways by which BIFs form, and their possible use as proxies for ambient environments and/or microbial processes, we studied the largest interval of BIF deposition, the 2.5 Ga BIFs, including the Dales Gorge member of the Brockman Iron Formation (Australia) and the Kuruman Iron Formation (South Africa). These studies ranged from the micron scale, via in situ O and Fe stable isotope analysis, to the 100’s of meters scale, through studies of the stratigraphic changes from shallow- to deep-water deposition.
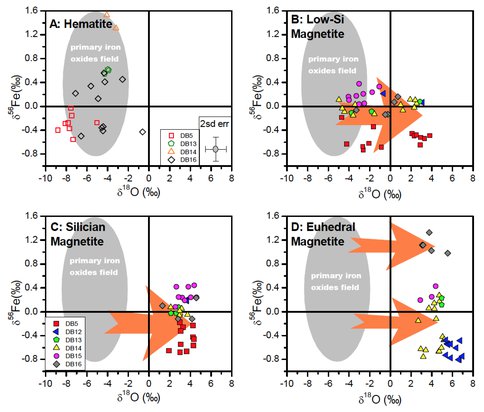
Combined in situ O and Fe isotope analyses of magnetite and hematite document large variations in both O and Fe isotope compositions in the Fe oxides. Hematite has consistently low δ18O values that range from -8.8 ‰ to -0.6 ‰. Three subgroups of magnetite, identified based on texture, composition, and BSE images, have different O isotope compositions. Euhedral magnetite and silician magnetite have high δ[^18^O values, ranging from 2.7 ‰ to 7.0 ‰, and 1.3 to 4.6 ‰, respectively, whereas low-Si magnetite cores of magnetite grains, or layers, have lower δ18O values, ranging from -5.6 to 3.8 ‰. Large O isotope zoning between silician magnetite overgrowths and low-Si cores exists at mm-scales within single magnetite grains or layers. The variable δ18O values of magnetite grains reflect variable degrees of isotopic exchange with fluids and variable temperatures up to ~300 °C during post-depositional processes. In contrast to the common occurrence of fine-scale O isotope zonation, in situ analyses show Fe isotope homogeneity to ±0.2 ‰ for 56Fe/54Fe ratio in magnetite at sub-cm scales, regardless of the difference in textures and O isotope variations among subgroups of magnetite. Although such homogeneity might be explained by fast diffusion of Fe in magnetite at <300 ºC during metamorphism, there is a >2 ‰ variation in δ56Fe values between the Dales Gorge member BIF samples, indicating isotope heterogeneity at scales greater than centimeters. The large variation in δ56Fe values of iron oxides is interpreted to reflect Fe isotope variability of Fe(III) hydroxide precipitates produced through variable extents of oxidation and oxidation/precipitation from aqueous Fe(II) of variable isotopic compositions depending on the proportion of hydrothermal and microbial Fe(III) reduction sources. In samples that have coexisting hematite and magnetite, the δ56Fe values of magnetite follow those of hematite but are consistently lower by 0.3 ‰ on average, over a δ56Fe range of -0.56 to +1.53 ‰ for hematite. This relation implies that magnetite to a large extent inherited the Fe isotope compositions of the Fe(OH)3 precursors, as may occur during microbial Fe(III) reduction when Fe(II)aq contents are low, but small extents of isotopic exchange during and after magnetite formation has slightly shifted the magnetite-hematite pairs towards isotope equilibrium.
Combined in situ O and Fe isotope analyses highlight the contrasting behavior of O and Fe isotopes in hematite and magnetite in BIFs (Figure 1). Oxygen isotope variations in iron oxides of BIFs are mainly controlled by processes during and after early diagenesis, and we can use in situ O isotope analysis to study post-depositional fluid mineral interactions on BIFs. In contrast, Fe isotope variations in iron oxides of BIFs are mainly controlled by processes that occurred prior to burial metamorphism, and in situ Fe isotope analysis provides insight into Fe pathways in the photic zone and the soft sediments during early diagenesis, prior to significant burial metamorphism. Therefore, in situ O and Fe isotope analyses are a powerful combination that provides a deeper understanding of BIF genesis than that of the individual isotopic systems by themselves, providing new constraints on the use of BIFs as proxies for paleoenvironmental conditions and microbial processes.
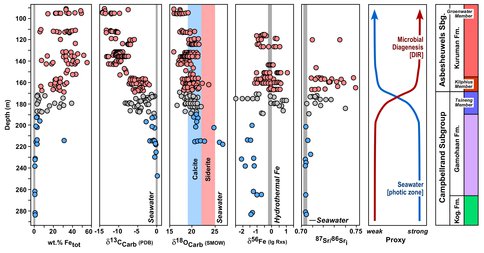
Turning to large, stratigraphic-scale variations, a second component of the study investigated the temporal transition from shallow- to deep-water deposition of the Campbellrand carbonate platform, South Africa (Figure 2). The transition to deep-water, Fe-rich deposition is clearly accompanied up section by C isotope compositions that move away from those appropriate for precipitation in equilibrium with seawater toward those that are dominated by organic sources of carbon, indicating heterotrophic respiration. The decrease in δ13C values is accompanied by decreasing δ18O values, which, when considering the distinct 18O/16O fractionations between Ca-Mg and Fe-rich carbonates, highlights that the δ18O values of BIF carbonates are far from those expected for seawater. The unusually low-δ18O values of BIF carbonates is interpreted to reflect inheritance from precursor Fe(III) oxides that were reduced by DIR, and the stratigraphic correlations between δ13C and δ18O values. δ56Fe values for Ca-Mg carbonates are consistently low, reflecting a seawater Fe(II) pool that is residual to the extensive BIF deposition that occurred elsewhere in the basin. With the transition to Fe-rich deposition on the platform during marine transgression, δ56Fe values shift to an average value of zero, as required for hydrothermally-sourced Fe, but the spread in δ56Fe values becomes large, recording extensive meter-scale microbial cycling of Fe in the soft sediment prior to lithification. These changes are accompanied by a dramatic increase in spread of initial 87Sr/86Sr ratios for BIF carbonates, far greater than the relatively small spread measured for Ca-Mg carbonates; the Sr isotope data provide strong evidence that although the Ca-Mg carbonates deposited in shallow water reflect equilibrium with seawater, the highly radiogenic Sr isotope compositions of the BIF carbonates clearly demonstrate that they are not in equilibrium with seawater.
Collectively, the transitions in chemical and isotopic compositions in Figure 2 paint a consistent story: the transition from Ca-Mg carbonate to Fe-rich carbonate was accompanied by a change in the nature of what these geochemical proxies record. Ca-Mg carbonates most closely record shallow (photic zone) seawater chemistry and surface-environmental conditions, whereas Fe-rich carbonates are dominated by microbial diagenesis – essentially the last thing the rocks “saw” before lithification. This reflects the fact that Fe(III) oxide formation and CO2 fixation to organic carbon in the photic zone, produced either from oxygenic or anoxygenic photosynthesis, supplied the reactants required to support extensive DIR on the seafloor. These results suggest that BIF carbonates, at least those studied here, are excellent proxies for microbial processes, and cannot, therefore, be used as a direct constraint on the chemical or isotopic compositions of the open ocean, or surface paleoenvironments, including past atmospheric carbon dioxide levels, as proposed by other workers.
Publications
-
Johnson, C. M., Ludois, J. M., Beard, B. L., Beukes, N. J., & Heimann, A. (2013). Iron formation carbonates: Paleoceanographic proxy or recorder of microbial diagenesis?. Geology, 41(11), 1147–1150. doi:10.1130/g34698.1
-
Li, W., Huberty, J. M., Beard, B. L., Kita, N. T., Valley, J. W., & Johnson, C. M. (2013). Contrasting behavior of oxygen and iron isotopes in banded iron formations revealed by in situ isotopic analysis. Earth and Planetary Science Letters, 384, 132–143. doi:10.1016/j.epsl.2013.10.014
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Clark Johnson
Project Investigator
Brian Beard
Co-Investigator
Nicolas Beukes
Collaborator
Adriana Heimann
Collaborator
Jason Huberty
Collaborator
Noriko Kita
Collaborator
Weiqiang Li
Collaborator
James Ludois
Collaborator
John Valley
Collaborator
-
RELATED OBJECTIVES:
Objective 2.1
Mars exploration.
Objective 4.1
Earth's early biosphere.
Objective 5.2
Co-evolution of microbial communities
Objective 6.1
Effects of environmental changes on microbial ecosystems
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems