2013 Annual Science Report
 University of Illinois at Urbana-Champaign
Reporting | SEP 2012 – AUG 2013
University of Illinois at Urbana-Champaign
Reporting | SEP 2012 – AUG 2013
Thermodynamics of Life
Project Summary
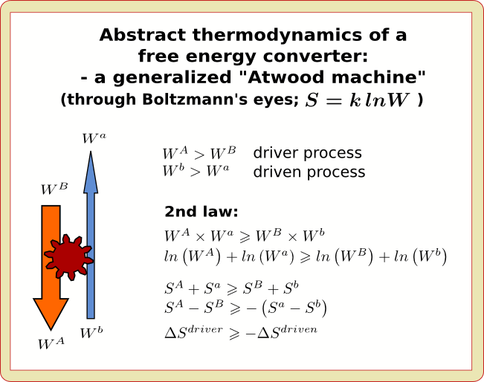
Although thermodynamics dictates that all spontaneous processes must be purely dissipative and “destructive” (the notoriously ungenerous face of the “2nd law”), under particular circumstances a spontaneous process can be a compound of two mechanistically coupled sub-processes only one of which (necessarily the larger one), is dissipative while its coupled, lesser partner is literally “driven” to be creative and generative – that is, a process that can “do work”, “build stuff”, and “make things happen”. A system functioning in this way is technically an engine and all living systems are necessarily, examples of such thermodynamically compound and creative “engine” systems – while at the same time operating internally via a complex, interlinked clockwork of such engines.
Moreover, living systems inherently belong to a special thermodynamic subclass of such engines, namely those that are “autocatalytic” (self-growing and self-stabilizing) in their operation. Arguably, in fact, it is the property of being autocatalytic thermodynamic engines which at root underlies the potency and magic of living systems and which at the same time constitutes life’s most assuredly universal, fundamental, and primitive property. However, as of yet, we understand the implications of these thermodynamic facts quite poorly – notwithstanding that they seem certain to materially impact questions regarding the origin of life, evolutionary dynamics, and community, trophic, and ecology-level organization.
The present project undertakes to redress this situation to some extent by investigating the formal dynamical behavior of model systems made up of interacting, thermodynamically driven, autocatalytic engines.
Project Progress
The main progress made thus far toward the goals of this project has been in collaterally motivated (and previously initiated) work which, however, bears on questions of foundational relevance to the present project’s direct goals. These efforts have sought first to clarify the thermodynamic and mechanistic properties of the “free energy conversion” processes (i.e. those mediated by “engines”) which underlie the energy metabolism of extant living systems. And second, based on that, they have sought to develop testable hypotheses about the abiotic/mineralogic analogues to these free energy conversion processes which, my collaborators and I argue, must have acted to initiate the emergence of life on earth. This work, carried out primarily with Michael Russell of JPL, but also with Wolfgang Nitschke at the Bioénergétique et Ingéniere des Protéines, CNRS, has thus far led to two published papers (see below), a third that is at the moment in the final steps of acceptance for publication in Astrobiology, and a further paper currently under construction.
Also, I presented, by invitation, two meeting lectures during the period under review. One was the opening lecture at the NAI-sponsored meeting on “Engines of Life: Thermodynamic Pathways to Metabolism”, at the Beyond Center at Arizona State University held in April, 2013 (talk title: “Life and the Twin Devices of Disequilibrium Management: Engines and Catalysts”). The second was a remote lecture to the NAI-sponsored “Thermodynamics, Disequilibrium, and Evolution” Focus group during the group’s October, 2013 meeting in Italy. Talk title: “Transcending Chemistry; the Janusian devices that keep life running.”
In addition, I’ve made what I regard as significant progress on the first of the directly specified goals in the present project:
1. Are living systems, as modeled as Cottrell systems, governed by a principle of thermodynamic optimality; that is, does there exist a thermodynamically defined, functionally optimal, dynamic state which is an attractor for the dynamics of Cottrell systems?
However, although this preliminary work is fairly complete in itself and provides, within the constraints of its assumptions, an answer to the question posed, the model on which it is based is not yet of sufficient generality to be of inarguable relevance to living systems. Achieving the desired model generality is the current challenge.
Publications
-
Branscomb, E., & Russell, M. J. (2013). Turnstiles and bifurcators: The disequilibrium converting engines that put metabolism on the road. Biochimica et Biophysica Acta (BBA) – Bioenergetics, 1827(2), 62–78. doi:10.1016/j.bbabio.2012.10.003
-
Russell, M. J., Nitschke, W., & Branscomb, E. (2013). The inevitable journey to being. Philosophical Transactions of the Royal Society B: Biological Sciences, 368(1622), 20120254–20120254. doi:10.1098/rstb.2012.0254
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Elbert Branscomb
Project Investigator
-
RELATED OBJECTIVES:
Objective 3.3
Origins of energy transduction
Objective 3.4
Origins of cellularity and protobiological systems
Objective 4.2
Production of complex life.
Objective 5.1
Environment-dependent, molecular evolution in microorganisms
Objective 5.2
Co-evolution of microbial communities