2013 Annual Science Report
 Carnegie Institution of Washington
Reporting | SEP 2012 – AUG 2013
Carnegie Institution of Washington
Reporting | SEP 2012 – AUG 2013
Project 4: Geochemical Steps Leading to the Origins of Life
Project Summary
We investigate the geochemical steps that may have lead to the origin of life, focusing on identifying and characterizing mineral catalyzed organic reaction networks that lead from simple volatiles, e.g., CO2, NH3, and H2, up to greater molecular complexity. We continue to explore the role of minerals to enhance molecular selection, both isomeric and chiral selection, as well as molecular organization on mineral surfaces. We continue to refine our understanding of the evolution of mineralogical complexity in the context of planetary evolution.
Project Progress
Project 4. Geochemical Steps Leading up to the Origins of Life
4.1
Post Doctoral Researcher Ohara and PI Cody have continued to explore the conditions to sustain a dynamic organic reaction network (DORN). We recognize that in order for interesting organic chemistry to arise that may lead toward greater chemical complexity and possibly developing the potential for the emergence of life, environments where recursive and stable organic reaction networks must operate. Demonstration of any single reaction is not sufficient. Over the past year Ohara and Cody along with collaborators at George Mason University (H. Morowitz, V. Singh, and E. Smith) have been exploring systems capable of molecular growth through sequential, often competitive reactions. One particularly interesting system involves reactions of glycolic and pyruvic acids with transitional metals at moderate temperatures (T = 80 °C) and ambient pressures. We have identified a very promising route into the central metabolism leading to many of the constituents of the Tricarboxylic Acid (TCA) Cycle. A manuscript describing this research is near ready for submission.
4.2
Post Doctoral Researcher Glein and PI Cody have been focusing on the kinetics and mechanism of carboxyl exchange with aqueous CO2 and/or bicarbonate (HCO3-). It has long been reported that isotopic exchange can occur between dissolved carbonate and soluble organic carboxylic acids, but the mechanism has not been resolved. This reaction is important in that it may provide an important control on stable isotope concentrations of carboxylic acids in the natural environment (e.g. in sedimentary basins) and also extraterrestrial environments (e.g. the interiors of primitive bodies). It is also important as CO2 addition to organic substrates is an essential biochemical reaction, one that has not been demonstrated abiologically. Glein and Cody have been performing aquathermolytic reactions over a wide range of times, temperatures and pH with molecular substrates that can be systematically perturbed via positional substitution to aid in deducing mechanism. We are very near finishing these experiments and plan to submit a paper on these experiments early in 2014.
4.3
During the summer of 2013, High School Science Intern Robert Martin working with Post Doc Glein and PI Cody began a study into identification of plausible routes towards partial oxidation of saturated organic molecules. One of the keys to developing molecular complexity in dynamic organic reaction networks (DORNs) is the activation of otherwise stable molecules to enable subsequent organic reaction. Investigating a range of potential weak oxidants Martin was able to find a class of transition metal sulfides well suited for weak oxidation, for example oxidizing succinic acid to fumaric acid. Martin submitted this work to the Siemens Science Competition and was made a semi-finalist. We are continuing this work and intend to prepare a paper for publication.
4.4 Extent and Mechanisms of Molecular Adsorption onto Mineral Surfaces
Biomolecule attachment to mineral surfaces is a topic of fundamental importance in a wide range of topics, including biomineralization, adhesives, medical implants, and the role of mineral-molecule interactions in the origin of life. Studies by CoIs Hazen and Svergenski in association with predoctoral student involvement have focused on the adsorption of amino acids, sugars, and nucleic acid fragments on common rock-forming minerals. We have studied adsorption of molecules under wide ranges of environmental conditions, including pH, ionic strength, competing solute species, and surface loading, by employing batch adsorption and potentiometric titration methods. Modeling of the data with the extended triple-layer model indicates the importance of different modes of attachment of the molecules to mineral surfaces. For example, glutamate and aspartate adsorb at low pH and low surface coverage with four points of attachment, i.e. lying down on the surface. However, at pH values of 5 to 7 they adsorb primarily with two points of attachment through the oxygen atoms of the carboxylate on the side-chain, i.e. standing up. These different modes of attachment and their dependence on environmental conditions are important for understanding the potential reactivity of the adsorbed molecules in geochemical and industrial processes.
- We have discovered that biomolecules commonly display 2 or more geometries of adsorption on mineral surfaces, including competing “standing up” and “lying down” geometries.
- We have extended our studies to several relevant rock-forming minerals, including brucite [Mg(OH)2], diopside (CaMgSi2O6), and clays.
- We have extended our studies to key biomolecules, including pentose sugars, nucleotides, and nucleotides.
- We have demonstrated competitive adsorption phenomena, for example in the selective adsorption of ribose from a mixture of pentose sugars on the surfaces of rutile.
- We have demonstrated cooperative adsorption phenomena, for example in the adsorption of aspartate and Ca2+ ions on the surfaces of brucite.
4.5 Mineral Evolution and Origins of Life Research
A central motivation of our research continues to be the geochemical context and constraints of the origins of life. CoIs Hazen and Svergenski are fascinated by the key roles that mineral surfaces may have played in life’s emergence through the selection, concentration, and organization of life’s essential building blocks. The work of our lab and elsewhere has shown that mineral surfaces also promote organic synthesis reactions that mimic life’s chemistry. Therefore, a central objective of our origin-of-life research program has been to develop experimental and theoretical protocols for understanding interactions between mineral surfaces and organic molecules. These studies rely, in part, on identifying which mineral species are plausible participants in prebiotic chemical processes. We have thus been applying the insights of “mineral evolution”—the study of Earth’s changing near-surface mineralogy through deep time—in our origins and mineral surfaces work. Key findings include:
- Geochemical environments are far more complex than those assumed in most origins of life scenarios. Fluxes, gradients, cycles, surfaces, and chemical complexity are critical to understanding the emergence of life.
- Prebiotic Earth had a limited mineralogical inventory of an estimated 420 species—only about 8% of today’s diversity. Some minerals invoked in origins models may not have been available.
- Clay minerals, which are often invoked in origins scenarios, were common prebiotic phases, but the distribution of clay minerals was dominated by Mg-Fe trioctahedral clay species, and thus was significantly different from today.
4.6 Characterizing Mineral Surfaces
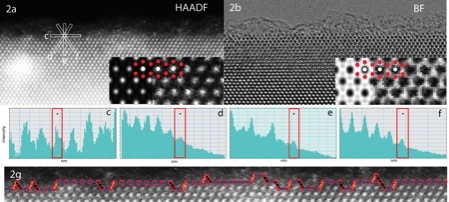
Efforts to understand the interactions between biomolecules and mineral surfaces depend on accurate characterizations of surfaces at the atomic scale. CoIs Hazen and Svergenski along with graduate students have engaged in a variety of studies to elucidate surface roughness, ion exchange, and reactivity in a variety of aqueous environments. Key findings include:
- The surface of rutile is far rougher than previously assumed, with almost one-third of all sites on the “{110}-type surfaces” possessing (111)-type atomic sites.
- The surface of calcite (CaCO3) is dynamic, with significant ion exchange at the surface.
4.7 Studies of Hydrothermal Systems
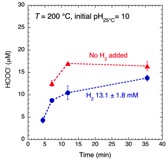
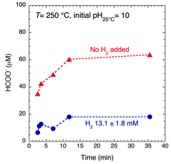
A number of models for life’s origins focus on elevated temperature and pressure conditions, such as those found at deep-sea hydrothermal vent systems. However, serious objections have been directed at such models because of the assumed instability of key biomolecules at extreme conditions. Accordingly, CoIs Hazen and Svergenski, PI Cody and collaborator Foustoukos have worked with a graduate student to begin experimental studies of biomolecules under high pressure and temperature. A key finding is that the stability of glutamic acid at elevated temperature is dramatically increased under reducing conditions where H2 is present in the aqueous phase—a situation common in deep ocean zones of basalt weathering and serpentinization.
Publications
-
Hazen, R. M., & Schiffries, C. M. (2013). Why Deep Carbon?. Reviews in Mineralogy and Geochemistry, 75(1), 1–6. doi:10.2138/rmg.2013.75.1
-
Hazen, R. M., Downs, R. T., Jones, A. P., & Kah, L. (2013). Carbon Mineralogy and Crystal Chemistry. Reviews in Mineralogy and Geochemistry, 75(1), 7–46. doi:10.2138/rmg.2013.75.2
-
Hazen, R. M., Downs, R. T., Kah, L., & Sverjensky, D. (2013). Carbon Mineral Evolution. Reviews in Mineralogy and Geochemistry, 75(1), 79–107. doi:10.2138/rmg.2013.75.4
-
Hazen, R. M., Golden, J., Downs, R. T., Hystad, G., Grew, E. S., Azzolini, D., & Sverjensky, D. A. (2012). Mercury (Hg) mineral evolution: A mineralogical record of supercontinent assembly, changing ocean geochemistry, and the emerging terrestrial biosphere. American Mineralogist, 97(7), 1013–1042. doi:10.2138/am.2012.3922
-
James Cleaves II, H., Michalkova Scott, A., Hill, F. C., Leszczynski, J., Sahai, N., & Hazen, R. (2012). Mineral–organic interfacial processes: potential roles in the origins of life. Chem. Soc. Rev., 41(16), 5502. doi:10.1039/c2cs35112a
-
Lee, N., Hummer, D. R., Sverjensky, D. A., Rajh, T., Hazen, R. M., Steele, A., & Cody, G. D. (2012). Speciation of l -DOPA on Nanorutile as a Function of pH and Surface Coverage Using Surface-Enhanced Raman Spectroscopy (SERS). Langmuir, 28(50), 17322–17330. doi:10.1021/la303607a
- Hazen, P.G., Carney, J.F. & Evangelista, E. (1989). Angiolymphoid Hyperplasia with Eosinophilia – Removal with Carbon-Dioxide Laser in a Patient on Chronic Oral Anticoagulants. Cutis, 44(2): 147-150.
- Kasem, K.K., Hazen, R. & Spaulding, R.M. (2002). Electrochemical studies on substituted iron-hexacyanoiron(III) bi-layered thin films at glassy carbon electrode/electrolyte interface. Interface Science, 10(4): 261-269. doi:Doi 10.1023/A:1020848711982
- Ohara, S., Srinivasan, V., Smith, E., Morowitz, H.J. & Cody, G.D. (2014). The Abiotic Synthesis of alpha-keto glutarate and other compounds from glyoxylate and pyruvate promoted by transition metal ions. Proceedings of the National Academey of Science, In Press.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Nabil Boctor
Co-Investigator
Henderson Cleaves
Co-Investigator
Dionysis Foustoukos
Co-Investigator
Katerina Klotchko
Co-Investigator
Nami Lee
Co-Investigator
Shohei Ohara
Co-Investigator
Codi Zarar
Co-Investigator
Donald Sparks
Collaborator
-
RELATED OBJECTIVES:
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules


