2012 Annual Science Report
 Carnegie Institution of Washington
Reporting | SEP 2011 – AUG 2012
Carnegie Institution of Washington
Reporting | SEP 2011 – AUG 2012
Project 4: Geochemical Steps Leading to the Origins of Life
Project Summary
The origins of life on Earth remains one of the outstanding problems in science. This project seeks to go to the root of the problem and focus on what were likely critical first steps. The research focuses on the natural synthesis of small organic molecules and subsequent interaction with potentially catalytic mineral phases opening up the system to greater chemical complexity. Organic mineral interactions are complex and difficult to analyze. Using a variety of powerful spectroscopic and mass spectrometric tools we are able to perform experiments that yield data that aid our understanding of such interactions.
Project Progress
Project 4: Geochemical steps leading to origins of life
4.1 The Geochemical Roots of life
Post Doctoral fellow Shohei Ohara working with PI Cody continued their studies of organic-mineral interactions. Focusing on transition metal sulfide catalyzed formation of oligo peptides and citrate FeS interactions. In the former case they have found that at near ambient temperatures, ZnS catalyzes the polymerization of glycine to form simple oligopeptides. The catalytic mechanism is not known for certain, but is inferred to be derived from selective adsorption of glycine on the surface of ZnS, enhancing the local concentration of glycine far above solution concentration, thus favoring the spontaneous condensation reaction. In a second set of reaction involving citric acid and FeS it was discovered that considerable dissolution of FeS occurs at near ambient temperatures; the iron-citrate clusters spontaneously react to form pyruvate through an iron catalyzed retro-aldol reaction. This work was presented at the AbSciCon 2012 meeting held in Atlanta, GA, and a manuscript based on this work is in preparation.
Post Doctoral fellow Codi Lazar working with PI Cody and collaborator Craig Manning (UCLA) completed their work on pressure catalyzed methanogenesis. In this set of experiments is found that in the absence of catalysts, increased pressure strongly increases the rate of methane formation from CO2 and H2. This work has been submitted for publication.
4. 2 Prebiotic molecular selection
CoI’s Hazen and Sverjensky continued their studies of mineral-molecule interactions by employing a diverse and expanded arsenal of experimental and theoretical tools to understand the molecular-scale behavior of small biological molecules. Our research is predicated on the recognition that interactions of aqueous organic molecules with mineral surfaces are of fundamental interest in a wide range of technological and scientific topics from the safety of human medical implants to the origins of life on Earth. Here we describe 5 research projects that have come to fruition during the past 12 months.
4.2.1. Why Ribose: Competitive Adsorption of Pentose Sugars on Rutile (TiO2):
Pentose sugars and amino acids are important major bio-building blocks. By what mechanism were these critical molecules selected and concentrated prior to life’s origins? The pentose sugars ribose, xylose, lyxose and arabinose differ only in the arrangement of the OH-groups and their stereochemistry in solution, which might contribute to selective adsorption on mineral surfaces. Post Doc Klotchko working with CoI’s Hazen and Sverjensky focused on interactions of pentose sugars on rutile (a-TiO2, pHPPZC = 5.4, BET = 18.1 m2/g) in pure water, 10 and 100 mM NaCl and synthetic seawater solutions with 5 < pH < 10. Batch adsorption experiments of the four sugars individually and in mixtures indicate that adsorption increases with increasing pH and salt concentration of the solution. The salt effect and pH dependancy of adsorption is more pronounced for ribose. Ribose adsorption is the strongest among the sugars, suggesting that ribose’s cis diol OH-groups play a critical role in the attachment to mineral surfaces.
This result conforms to our other recent experimental surveys of adsorption of bases, nucleosides and nucleotides on rutile, which indicate that adjacent OH-groups on an organic molecule can enhance its attachment to mineral surfaces. The isomeric pentose sugars (C5H10O5) differ by the arrangement of OH-groups and the proportion of molecule that exists in cyclic form in solution. In the first set of experiments by Klochko we focused on interactions of D-ribose and D-xylose on rutile surface (a-TiO2, pHPPZC = 5.4, BET = 18.1 m2/g) in 10 and 100 mM NaCl solutions over a wide range of pH conditions (3-10). The rutile powder, obtained from Oak Ridge National Laboratory, is ideal for this study as it is highly insoluble, has no ion exchange characteristics, has been extensively studied and is very well characterized.
The results of the batch adsorption experiments of D-ribose and D-xylose on rutile individually and in mixtures indicate that adsorption of both sugars increases with increasing pH of the starting solution. pH dependancy of adsorption is more pronounced in ribose. Moreover, D-ribose adsorbs more strongly than D-xylose, with the maximum effect of ~20% in pH conditions higher than 8.5 (Figure 1).
In dark conditions, batch adsorption experiments of the four sugars individually and in mixtures indicate that adsorption increases with increasing pH and salt concentration of the solution. The salt effect and pH dependency of adsorption is more pronounced for ribose. Ribose adsorption is the strongest among the sugars, suggesting that ribose’s cis diol OH-groups play a critical role in the attachment to mineral surfaces (Figure 2).
4.2.2. Detection of surface species on minerals using in situ spectroscopy:
Our previous studies reported adsorption of the catecholic amino acid 3,4-dihydroxyphenylalanine (DOPA) on rutile particles at various environmental conditions combined with a surface complexation model establishing the stoichiometry, model surface speciation, and thermodynamic equilibrium constants. At pH~3 a model species involving four attachment points (“lying down”) was suggested to predominate, whereas at pH~6 a species involving two attachment points via the phenolic oxygens (“standing up”) predominated (Figure 3). Lee and coworkers have conducted in situ SERS measurements to probe the attachment configurations of DOPA on nano-rutile at different pH values. The enhancement signals arise when a charge transfer complex forms between the nanoparticles and adsorbed DOPA. Our SERS spectra show signals that progressively change between pH values of 3 to 6 (Figure 4), indicating the change from the “lying down” to “standing up”, thereby validating the model predictions of two different surface complexes. The surface complex-specific signals provide a superb scheme for studying how the adsorption of molecules on nanoparticles in water changes with environmental conditions.
4.2.3. Experimental evidence that redox state influences amino acid stability under hydrothermal conditions:
With the discovery of an extensive biosphere near deep-sea hydrothermal vents, the stability of amino acids at elevated temperatures and pressures is of great interest for the cycling of C and N within the crust and the overlying oceanic water column. Previous studies provide strong evidence that the decomposition properties of amino acids are very sensitive to parameters such as temperature, and catalytic reactor surfaces. However, despite the clear relevance, the redox state of the system has rarely been controlled in such studies. Here hydrothermal experiments were conducted to investigate the influence of redox conditions on the stability of glutamic acid at pressures and temperatures reflecting near-seafloor hydrothermal environments (100–250 °C, 136 bar). Reactions were conducted for 3 to 35 min. in a titanium flow-through cell. The oxidation state was controlled by equilibrating ~ 13 mmolal of H2(aq) with a glutamic acid solution with an initial pH of ~10 at 25 °C. The products were identified and analyzed using gas chromatography and ionic chromatography with conductivity and electrochemical detectors. Results indicate that the reaction kinetics of glutamic acid under hydrothermal conditions is associated with conversion to the cyclic pyroglutamate via a dehydration reaction. Other products of glutamic acid included CO2(aq) and formate. It can be seen in Figures 5a and b that the decomposition of glutamic acid is strongly inhibited by the redox state imposed by the dissolved H2.
4.2.4. What Minerals were Present at the Origins of Life?
Recent estimates suggest that prebiotic Earth held no more than about 500 different mineral species, compared to more than 4500 recognized types today. This mineralogical parsimony is a consequence of the relatively limited modes of mineral paragenesis at 4.0 Ga compared to the last 3.0 billion years. In particular, the influences of subduction zone volcanism and associated massive sulfide deposition; the formation of complex pegmatites and hundreds of accompanying rare minerals of pegmatophile elements; and, most importantly, the influences of life itself, may be responsible for as many as 4000 of the known species. Consequently, any scenario for life’s origins that invokes minerals as agents of molecular synthesis, selection, protection or organization must take into account the limited mineralogical repertoire of the time.
In spite of this constraint, most of the minerals previously cited in origins-of-life research are plausible participants in prebiotic reactions. In particular, clay minerals, so often employed in origins studies for their ability to adsorb and template organic molecules, would have been present, though the distribution of clay mineral species was significantly different from the modern world. In particular, serpentinization would have produced abundant serpentine and talc, as well as brucite, while anoxic weathering of volcanic deposits may have yielded montmorillonite and kaolinite. This suite of clay minerals contrasts with the modern production, which is dominated by terrestrial clay minerals through oxic and biological weathering. Most of the rock-forming silicates, including Fe-Mg olivine, pyroxene, amphibole, and mica, as well as all major varieties of Na-K-Ca feldspar and feldspathoid would have been present, as well. Though volumetrically much less important, reactive Fe-Ni phases, including metal alloys, sulfides, and the phosphide schreibersite were all available continuously in the near-surface environment in the form of iron meteorites. By contrast, quartz, carbonates, and phosphate minerals, though present, appear to have been volumetrically insignificant, and it is not obvious that any borate minerals were available prior to 3.5 Ga.
4.2.5. Probing the interactions between glutamic acid and diopside:
The manner in which organic molecules adsorb onto the mineral/water interface may hold implications for the origin of life. Chiral amino acids may have selectively adsorbed onto the chiral growth faces of silicate minerals by three or more non-colinear points of attachment, which may have led to the evolution of homochirality. Diopside is a common clinopyroxene mineral with the chiral growth faces (110) and (1-10), which are also the principal cleavage planes prominent in powdered samples. We demonstrated with batch adsorption experiments that up to 30% of L-glutamic acid (initially 20 µM) adsorbs onto crushed natural diopside at pH=10. Estrada in collaboration with colleagues at Northwestern University investigated the enantioselective potential of diopside by probing the interactions between glutamic acid and the chiral growth faces (110) and (1-10) with vibrational sum frequency generation (SFG) spectroscopy. Prior to our experiment, we prepared two 0.63 mm thick (1-10) diopside sections of approximately 500 mm2, which were cleaned with a plasma cleaner. One diopside section was submerged in 100 µM L-glutamic acid for 2h and dried under nitrogen, whereas the other section served as a blank. The SFG spectra we collected for the diopside section with L-glutamic acid significantly differed from those of the blank. As a preliminary analysis, we assigned asymmetric and symmetric stretching modes for the β-methylene group and a symmetric stretching mode for the γ-methylene group of L-glutamic acid. We obtained SFG spectra of adsorbed L-glutamic acid under two experimental mutually perpendicular polarization combinations. In principle, spectra such as these can establish the configuration of the adsorbed molecule. A comparison of the ratio of SFG signal intensity for the methylene vibrational modes between the two polarization combinations will determine the dipole orientation of the methylene stretches relative to the diopside surface. This technique may be useful for establishing an enantioselective trend in glutamic acid adsorption.
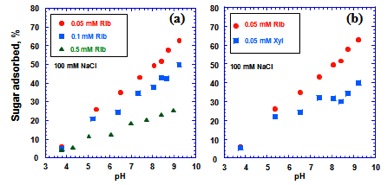
D-ribose adsorbs onto rutile (TiO2) more strongly than D-xylose.
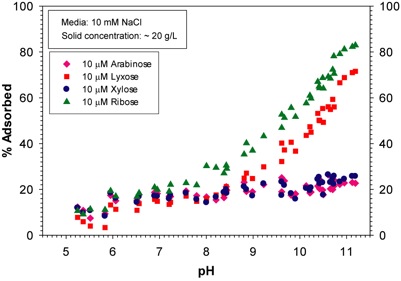
Competitive adsorption experiments of pentose sugars reveal that ribose adsorbs significantly more strongly than arabinose, lyxose, or xylose.
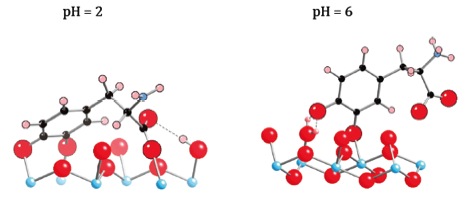
Proposed surface complexes of DOPA on rutile (Bahri et al., 2011). As a function of increasing pH the DOPA changes from the “lying down” to “standing up” adsorption mechanisms.
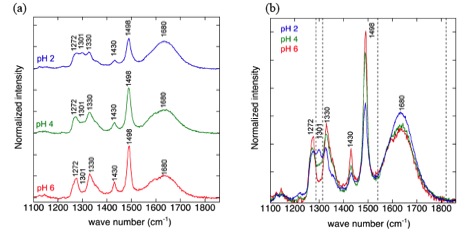
SERS detection of surface speciation changes for adsorbed DOPA on nanorutile in aqueous solution as a function of pH. In (a) and (b) as pH increases there is a progressive reduction of the peaks at 1301 cm-1 and 1680 cm-1.
Publications
-
Bahri, S., Jonsson, C. M., Jonsson, C. L., Azzolini, D., Sverjensky, D. A., & Hazen, R. M. (2011). Adsorption and Surface Complexation Study of L-DOPA on Rutile (α-TiO 2 ) in NaCl Solutions. Environ. Sci. Technol., 45(9), 3959–3966. doi:10.1021/es1042832
-
Grew, E. S., Bada, J. L., & Hazen, R. M. (2011). Borate Minerals and Origin of the RNA World. Orig Life Evol Biosph, 41(4), 307–316. doi:10.1007/s11084-010-9233-y
-
Hazen, R. M., Bekker, A., Bish, D. L., Bleeker, W., Downs, R. T., Farquhar, J., … Valley, J. W. (2011). Needs and opportunities in mineral evolution research. American Mineralogist, 96(7), 953–963. doi:10.2138/am.2011.3725
-
Hazen, R. M., Golden, J., Downs, R. T., Hystad, G., Grew, E. S., Azzolini, D., & Sverjensky, D. A. (2012). Mercury (Hg) mineral evolution: A mineralogical record of supercontinent assembly, changing ocean geochemistry, and the emerging terrestrial biosphere. American Mineralogist, 97(7), 1013–1042. doi:10.2138/am.2012.3922
-
James Cleaves II, H., Michalkova Scott, A., Hill, F. C., Leszczynski, J., Sahai, N., & Hazen, R. (2012). Mineral–organic interfacial processes: potential roles in the origins of life. Chem. Soc. Rev., 41(16), 5502. doi:10.1039/c2cs35112a
-
James Cleaves, H., Crapster-Pregont, E., Jonsson, C. M., Jonsson, C. L., Sverjensky, D. A., & Hazen, R. A. (2011). The adsorption of short single-stranded DNA oligomers to mineral surfaces. Chemosphere, 83(11), 1560–1567. doi:10.1016/j.chemosphere.2011.01.023
-
Parikh, S. J., Kubicki, J. D., Jonsson, C. M., Jonsson, C. L., Hazen, R. M., Sverjensky, D. A., & Sparks, D. L. (2011). Evaluating Glutamate and Aspartate Binding Mechanisms to Rutile (α-TiO 2 ) via ATR-FTIR Spectroscopy and Quantum Chemical Calculations. Langmuir, 27(5), 1778–1787. doi:10.1021/la103826p
- Hazen, R.M. & Papineau, D. (2012). Mineralogical Co-Evolution of the Geosphere and Biosphere [Book Chapter]. Fundamentals of Geobiology. John Wiley & Sons, Ltd.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Donald Sparks
Collaborator
Katerina Klotchko
Postdoc
Shohei Ohara
Postdoc
Codi Zarar
Postdoc
Nabil Boctor
Research Staff
Henderson Cleaves
Research Staff
Nami Lee
Graduate Student
Dionysis Foustoukos
Unspecified Role
-
RELATED OBJECTIVES:
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules


