2011 Annual Science Report
 VPL at University of Washington
Reporting | SEP 2010 – AUG 2011
VPL at University of Washington
Reporting | SEP 2010 – AUG 2011
Understanding Past Earth Environments
Project Summary
For much of the history Earth, life on the planet existed in an environment dramatically different than that of modern-day Earth. Thus, the ancient Earth represents a planet with a biosphere that is both dramatically different than the one in which we live and is accessible to detailed study. As such, is serves as a model for what types of biospheres we may find on other planets. A particular focus of our work was on the “Early Earth” (formation through to about 500 million years ago), a timeframe poorly represented in the geological and fossil records but comprises the majority of Earth’s history. We have studied the composition of the ancient atmosphere, modeled the effects of clouds on such a planet, studied the sulfur, oxygen and nitrogen cycles, and the atmospheric formation of molecules that were likely important to the origins of life on Earth.
Project Progress
The past year’s work on this task focused on the Earth’s history of feedbacks between the atmosphere, biosphere, and climate for planets with lower surface oxidation states than the modern Earth’s surface. There were three areas of focus. In the first, we examined the climatic impacts of N2O and CH4 (biogenic gases) for a planet with moderate amounts of O2 that are lower than modern-day values (Roberson et al., 2011). A second study used laboratory experiments to probe a potential feedback between sulfate levels, methane consumption, and the redox state of the environment (Beal et al., 2011). Finally, climatic effects of clouds during the Archaen were modeled (Goldblatt and Zahnle, 2011b).
Buildup of Nitrous Oxide, Hydrogen Cyanide, and Hydrogen Peroxide in the Early Earth’s Atmosphere: The team published a paper (Roberson et al., 2011) that examined the fluxes and climatic impacts of nitrous oxide (N2O) in the Earth’s Proerozoic atmosphere. An anoxic, sulfidic ocean that may have existed during the Proterozoic Eon (0.54-2.4 Ga) would have had limited trace metal abundances because of the low solubility of metal sulfides. The lack of copper, in particular, could have had a significant impact on marine denitrification. Copper is needed for the enzyme that controls the final step of denitrification, from N2O to N2. Today, only about 5-6 percent of denitrification results in release of N2O. If all denitrification stopped at N2O during the Proterozoic, the N2O flux could have been 15- 20 times higher than today, producing N2O concentrations of several ppmv, but only if O2 levels were relatively high (>0.1 PAL). At lower O2 levels, N2O is rapidly photodissociated. CH4 concentrations may also have been elevated during this time, as has been previously suggested. A lack of dissolved O2 and sulfate in the deep ocean could have produced a high methane flux from marine sediments, as much as 10-20 times today’s methane flux from land. The photochemical lifetime of CH4 increases as more CH4 is added to the atmosphere, so CH4 concentrations of up to 100 ppmv are possible during this time. The combined greenhouse effect of CH4 and N2O could have provided up to 10 degrees of warming, thereby keeping the surface warm during the Proterozoic without necessitating high CO2 levels. A second oxygenation event near the end of the Proterozoic would have resulted in a reductions of both atmospheric N2O and CH4, perhaps triggering the Neoproterozoic “Snowball Earth” glaciations.
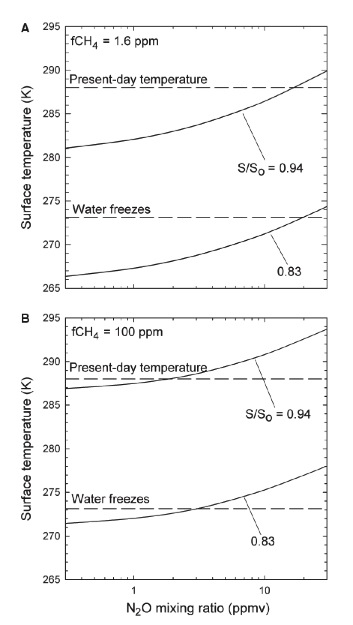
a) Global mean surface temperature (K) vs. nitrous oxide abundance. Two curves represent calculations for two solar luminosities: 83% and 94% of present value So. Concentration of CH4 is fixed at 1.6 ppm. (CO2 assumed at preindustrial value of ~320 ppm)
b) Global mean surface temperature (K) vs. nitrous oxide abundance at a higher methane concentration. Two curves represent calculations for two solar luminosities: 83% and 94% of present value So. Concentration of CH4 fixed at 100 ppm. (Figure from Roberson et al., 2011)
Another study on atmospheric chemistry was that of Haqq-Misra et al. (2011). In this work the availability of O2 and H2O2 on the pre-photosynthetic Earth was modeled. Old arguments that free O2 must have been available at Earth’s surface prior to the origin of photosynthesis have been revived by a new study that shows that aerobic respiration can occur at dissolved oxygen concentrations much lower than had previously been thought, perhaps as low as 0.05 nM, which corresponds to a partial pressure for O2 of about 4×10-8 bar. We used numerical models to study whether such O2 concentrations might have been provided by atmospheric photochemistry. Results show that disproportionation of H2O2 near the surface might have yielded enough O2 to satisfy this constraint. Alternatively, poleward transport of O2 from the equatorial stratosphere into the polar night region, followed by downward transport in the polar vortex, may have brought O2 directly to the surface. Thus, our calculations indicate that this “early respiration” hypothesis might be physically reasonable.
Finally, another paper (Tian et al., 2011) on this subtask examined the ability for hydrogen cyanide (HCN) to accumulate in a prebiotic atmosphere. HCN is a critical molecule to origin of life models and experiments. Thus, this paper’s improvement of estimates of global HCN production rates has a bearing not just on Earth history, but also on origin of life research.
Runaway Feedbacks on Archean Redox State Conventrations: This was a cross-team interdisciplinary astrobiology study (Beal et al., 2011) that made bench-top laboratory experiments to test theories proposed by Catling et al. in 2007. Microbes that can oxidize methane by reducing sulfate were cultivated at varying sulfate concentrations, and shown to continue metabolizing at much lower sulfate levels that previously anticipated. This strengthens a positive biological feedback on the rise in atmopsheric oxygen where increasing the oxidation state of the surface would increase seawater sulfate, in turn increasing the rate of methane oxidation. This would have decreased the sink for oxidants, thereby amplifying the oxidation of the surface.
Role of clouds in early Earth climate: The team published two papers (Goldblatt and Zahnle, 2011a and 2011b) that studied the effects of clouds on the early Earth’s climate. This is relevant to “the faint young sun paradox,” which is the juxtaposition of expectations that the sun was dimmer early in Earth’s history with geological evidence for a climate at least as warm as today’s. One of the largest uncertainties in models of this time is clouds: these have a large effect on both albedo and the greenhouse effect and changes relative to present could have caused either warming or cooling on early Earth. We conducted a major parametric study, in which we fully explored the phase space of changing clouds [CG4] (cited 4 times in the year since publication). In addition to constraining what radiative forcing changing clouds could possibly cause, this has permitted objective evaluation of the forcing from various hypothesized changes to clouds. For example, we were able to respond rapidly to a high profile proposal by Rosing et al. (Goldblatt and Zahnle, 2011a) that less low cloud would resolve the paradox, showing that this was not quantitatively plausible. We also showed that a long-standing assumption in early Earth climate models that clouds could be neglected from the atmosphere and replaced with an enhanced surface albedo leads to a systematic over-estimate of the strength of greenhouse gas forcings. A spin-off application of our work on Earth was in astronomy; we were able to show that representing clouds in brown dwarf stars as having partial coverage (cloudy and cloud free sub-columns in a single-column model) gave a much better fit to observed spectra than existing models (Marley et al., 2010).
Publications
-
Beal, E. J., Claire, M. W., & House, C. H. (2011). High rates of anaerobic methanotrophy at low sulfate concentrations with implications for past and present methane levels. Geobiology, None, no–no. doi:10.1111/j.1472-4669.2010.00267.x
-
Campbell, A. J., Waddington, E. D., & Warren, S. G. (2011). Refugium for surface life on Snowball Earth in a nearly-enclosed sea? A first simple model for sea-glacier invasion. Geophysical Research Letters, 38(19), n/a–n/a. doi:10.1029/2011gl048846
-
Goldblatt, C., & Zahnle, K. J. (2011). Clouds and the Faint Young Sun Paradox. Clim. Past, 7(1), 203–220. doi:10.5194/cp-7-203-2011
-
Goldblatt, C., & Zahnle, K. J. (2011). Faint young Sun paradox remains. Nature, 474(7349), E1–E1. doi:10.1038/nature09961
-
Haqq-Misra, J., Kasting, J. F., & Lee, S. (2011). Availability of O 2 and H 2 O 2 on Pre-Photosynthetic Earth. Astrobiology, 11(4), 293–302. doi:10.1089/ast.2010.0572
-
Marley, M. S., Saumon, D., & Goldblatt, C. (2010). A PATCHY CLOUD MODEL FOR THE L TO T DWARF TRANSITION. The Astrophysical Journal, 723(1), L117–L121. doi:10.1088/2041-8205/723/1/l117
-
Roberson, A. L., Roadt, J., Halevy, I., & Kasting, J. F. (2011). Greenhouse warming by nitrous oxide and methane in the Proterozoic Eon. Geobiology, 9(4), 313–320. doi:10.1111/j.1472-4669.2011.00286.x
-
Summons, R. E., Amend, J. P., Bish, D., Buick, R., Cody, G. D., Des Marais, D. J., … Sumner, D. Y. (2011). Preservation of Martian Organic and Environmental Records: Final Report of the Mars Biosignature Working Group. Astrobiology, 11(2), 157–181. doi:10.1089/ast.2010.0506
-
Tian, F., Kasting, J. F., & Zahnle, K. (2011). Revisiting HCN formation in Earth’s early atmosphere. Earth and Planetary Science Letters, 308(3-4), 417–423. doi:10.1016/j.epsl.2011.06.011
- Claire, M. & Kasting, J.F. (2010). Variations in the magnitude of non mass dependent sulfur fractionation in the Archean atmosphere. AGU Fall Meeting Abstracts, 14: 03.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
David Des Marais
Co-Investigator
Watson Gregg
Co-Investigator
Tori Hoehler
Co-Investigator
Victoria Meadows
Co-Investigator
Antigona Segura-Peralta
Co-Investigator
Shawn Domagal-Goldman
Postdoc
Colin Goldblatt
Postdoc
Jacob Haqq-Misra
Graduate Student
Jonathan Breiner
Undergraduate Student
Noe Khalfa
Undergraduate Student
Yuk Yung
Unspecified Role
-
RELATED OBJECTIVES:
Objective 1.1
Formation and evolution of habitable planets.
Objective 1.2
Indirect and direct astronomical observations of extrasolar habitable planets.
Objective 4.1
Earth's early biosphere.
Objective 4.2
Production of complex life.
Objective 5.1
Environment-dependent, molecular evolution in microorganisms
Objective 5.2
Co-evolution of microbial communities
Objective 6.1
Effects of environmental changes on microbial ecosystems






