2011 Annual Science Report
 VPL at University of Washington
Reporting | SEP 2010 – AUG 2011
VPL at University of Washington
Reporting | SEP 2010 – AUG 2011
The Long Wavelength Limit for Oxygenic Photosynthesis
Project Summary
Photosynthesis is process where plants and bacteria use solar energy to produce sugar and oxygen. It is also the only known process that produces signs of life (biosignatures) on a planetary scale. And, because starlight (or solar energy) is one of the most common sources of energy, it is expected that photosynthesis will be successful on habitable extrasolar planets. Our team is studying how photosynthetic pigments – the molecules that make photosynthesis possible – might function in unique or extreme environments on other planets. In our experiments, we use a bacteria called Acaryochloris marina to study how different photosynthetic pigments work. This bacterium is useful for our research because it uses a pigment known as chlorophyll d instead of chlorophyll a, which is more common on our planet. Chrolophyll a works well in Earth’s environment but, by studying chlorophyll d, we can begin to understand how photosynthesis might work on planets with different environments than Earth. So far, our research is revealing that photosynthesis can occur quite efficiently in environments that are very different from our planet.
Project Progress
NAI postdoctoral fellow Steven Mielke has been continuing measurements of the efficiency of photon energy storage by wavelength in whole intact cells of the cyanobacterium Acaryochloris marina in the lab of Co-I Dr. David Mauzerall. A. marina is the only known organism to have chlorophyll d (Chl d) which uses photons at wavelengths in the far-red (713-715 nm) and near-infrared (740 m), whereas all other oxygenic photosynthetic organisms use chlorophyll a (Chl a) with absorbance peaks at 680 nm and 700 nm. Using pulsed, time-resolved photoacoustics (PTRPA or PA), we have obtained detailed measurements indicating that A. marina shows efficiencies higher than or comparable to those in Chl a-utilizing organisms. These results imply that oxygenic photosynthesis can operate quite effectively at far-red/near-infrared wavelength and is not suffering from losses to back reactions, something that had been unknown up till now. This then extends what is plausible on extrasolar planets. A paper was published in 2011 in Biochimica et Biophysica Acta on this work, comparing measurements of the efficiency of PSII in A. marina at 710 nm and in the Chl a-utilizing cyanobacterium Synechococcus leopoliensis (UTEX 625) at 670 nm. Completion of full spectral measurements of efficiencies of both of the above organisms over 670-800 nm has been under way, with a paper on this work likely before the end of 2011.
In preparation for studies to distinguish the separate contributions of Photosystem I and Photosystem II (PS I and PS II) to the overall photosynthetic efficiency, extraction of purified complexes of PS I and II have been done in the lab of Co-I Blankenship, with assistance from Dr. Min Chen at the University of Sydney, NSW, Australia. Measurements of the purified complex efficiencies will be done in the coming year. PS I and PS II complexes of A. marina have been prepared by extracting purified membranes with 1% dodecyl maltoside detergent, followed by sucrose density gradient centrifugation and ion exchange chromatography.
Complementing these lab studies, NAI postdoctoral fellow Steven Mielke’s has theoretically modeled redox potentials along the electron transfer pathway in Photosystem II with Chl a versus Chl d, using the Multi-Conformer Continuum Electrostatics (MCCE) computer program of collaborator Dr. Marilyn Gunner, at City College of New York. The modeling has uncovered subtle differences in redox potentials when Chl a is replaced with Chl.d. A paper is currently in preparation. More detail on this work may be found under the summary for NASA Postdoctoral Program fellow Steven Mielke.
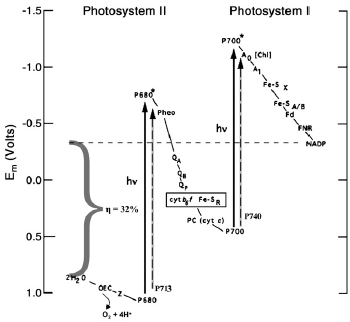
Z-scheme of oxygenic photosynthesis. Solid and dashed arrows represent, respectively, the changes in midpoint redox potential (Em) generated by photoexcitation of the primary donors in Chl a organisms (P680 in PSII and P700 in PSI), and in Acaryochloris marina (P713 in PSII and P740 in PSI). Because the ground- and excited state potentials of the latter are not well known, the placement of the dashed arrows is somewhat arbitrary. In Chl a species, the estimated maximum efficiency (η) of the overall reaction that oxidizes water and reduces NADP is indicated by the bracket, and is ≈32% of the total excitation energy of 3.60 eV, the sum of hν=1.83 eV for a 680-nm photon and 1.77 eV for a 700-nm photon. In A. marina, the corresponding efficiency is ≈33%, because both excitations are ≈5% lower in energy.
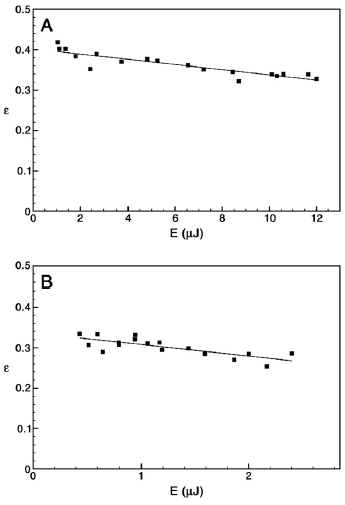
Energy-storage effciency, ε, as a function of laser pulse energy incident on samples containing (A) A. marina intact cells (pulse wavelength, λ=710 nm); and (B) S. leopoliensis intact cells (pulse wavelength, λ=670 nm). At low pulse energies, ε decreases linearly with increasing energy, so the maximal efficiency at chosen λ, εmax, is obtained from the y-intercept of a linear fit to efficiencies measured over a range of low energies. For A. marina, εmax =40±1%, and for S. leopoliensis, εmax =34±1%.
Publications
-
Mielke, S. P., Kiang, N. Y., Blankenship, R. E., Gunner, M. R., & Mauzerall, D. (2011). Efficiency of photosynthesis in a Chl d-utilizing cyanobacterium is comparable to or higher than that in Chl a-utilizing oxygenic species. Biochimica et Biophysica Acta (BBA) – Bioenergetics, 1807(9), 1231–1236. doi:10.1016/j.bbabio.2011.06.007
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Robert Blankenship
Co-Investigator
David Mauzerall
Co-Investigator
Marilyn Gunner
Collaborator
Steven Mielke
Postdoc
-
RELATED OBJECTIVES:
Objective 3.2
Origins and evolution of functional biomolecules
Objective 4.2
Production of complex life.
Objective 5.1
Environment-dependent, molecular evolution in microorganisms
Objective 5.3
Biochemical adaptation to extreme environments
Objective 6.2
Adaptation and evolution of life beyond Earth
Objective 7.2
Biosignatures to be sought in nearby planetary systems
