2011 Annual Science Report
 VPL at University of Washington
Reporting | SEP 2010 – AUG 2011
VPL at University of Washington
Reporting | SEP 2010 – AUG 2011
Postdoctoral Fellow Report: Steven Mielke
Project Summary
This project seeks to resolve the long-wavelength limit of oxygenic photosynthesis in order to constrain the range of extrasolar environments in which spectral signatures of biogenic oxygen might be found, and thereby guide future planet detecting and characterizing observatories.
Project Progress
As an NAI/NPP fellow, I have worked to resolve the long-wavelength limit of oxygenic photosynthesis to advance the goals of the VPL task described in the project report: “The Long-wavelength Limit for Oxygenic Photosynthesis” (Nancy Kiang, Project Lead). This year we are engaged in experimental and theoretical studies of photochemistry in Acaryochloris marina, a unique organism that employs an atypical photopigment-chlorophyll (Chl) d rather than Chl a-to perform oxygenic photosynthesis in far-red light environments [1].
Our experimental studies, carried out in collaboration with Prof. David Mauzerall (Rockefeller University) and supported by an NAI DDF grant, seek to elucidate thermodynamics of photochemistry in A. marina. Our experiments used pulsed, timeresolved photoacoustic (PA) spectroscopy, which is the only available means of directly measuring energy storage in photochemical systems [2]. Our specific goal is to obtain the in vivo, millisecond-timescale energy-storage efficiency in both A. marina and Synechococcus leopoliensis (a Chl a-utilizing control species) and to determine to what extent photosynthesis in A. marina is affected by its use of far-red (lower-energy) photons. Energystorage efficiency, ε, is defined as the ratio of energy stored in products of photochemistry to the photon energy absorbed.
Our principal conclusion to date is that the efficiency of photosynthesis in A. marina at its peak absorbance wavelength (710 nm)—i.e., ε = 40±1%—is comparable to or higher than that in oxygenic species that employ Chl a and inhabit typical light environments. This indicates that photosynthesis in Acaryochloris is not thermodynamically limited at wavelengths ≈40 nm longer than those utilized by typical organisms, and therefore oxygenic photochemistry generally is not fundamentally limited by these wavelengths. Thus, photosynthesis is likely viable in even redder light environments, such as those on planets in the HZ of M-dwarf stars.
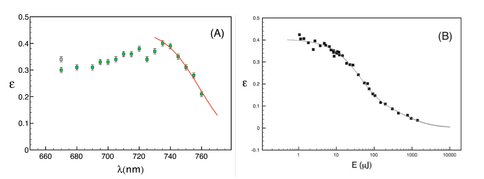
Progress during the present reporting period has included publication of these results in the journal Biochimica et Biophysica Acta [3] (see Fig. 2 in aforementioned project report), an completion of measurements of the efficiency in A. marina at wavelengths between 670 and 760 nm (Fig. 1A). Measurements of the efficiency as a function of wavelength in S. leopoliensis are currently underway. Analysis of these results (Fig. 1B) will provide detailed information on the specific contributions of photosystems (PS) I and II to overall energy storage and give us insights on how A. marina has adapted oxygenic photochemistry to far red light environments. A manuscript on the wavelength-dependence of the energy-storage efficiency is in preparation [4]. (Figure 1A, Figure 1B)
Progress has also included preliminary isolation and purification of A. marina photosystem complexes in the lab of project co-investigator, Prof. Robert Blankenship (Washington University). Purified PS I and PS II complexes will be employed in μs–ms-timescale PA studies. The results of this work will complement analyses of the in vivo results discussed above and provide information on (1) adaptive mechanisms at the level of fast-timescale photochemistry and (2) the crucial oxygen-forming process.
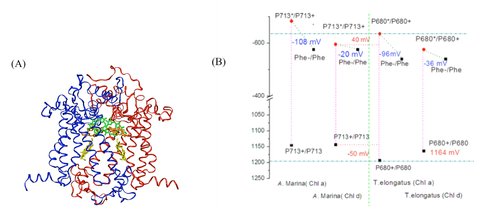
Our theoretical studies, carried out in collaboration with Prof. Marilyn Gunner (City College of New York), and supported in part by a DOE Energy Biosciences grant (Prof. Gunner, PI), seek to elucidate redox properties of photochemistry in A. marina using multi-conformation continuum electrostatics (MCCE) methods [5]. To date, we have: (1) using known A. marina protein sequences, developed a homology model of the organism’s PSII reaction center (D1 and D2 subunits), inclusive of the six core cofactors (Fig. 2A); (2) in both the A. marina model structure and a known Thermosynechococcus elongatus PSII crystal structure (PDB ID 3bz2), calculated Ems (midpoint redox potentials) of these cofactors using MCCE, where, in both structures, chlorophylls were designated alternately as Chl a or Chl d; and (3) compared the geometries of amino acid side-chains near the functional group that is modified in Chl d, relative to Chl a, in both the A. marina and T. elongatus structures (manuscript in preparation [6]). Our results indicate the Ems of the core cofactors in A. marina are not significantly different than those in T. elongatus, but that (1) the A. marina primary electron-donor ground-state (P713+) may be ~50 mV less oxidizing and the excitedstate (P713*) ~40 mV less reducing than the corresponding states in T. elongatus (Fig. 2B); and (2) preference for binding Chl d over Chl a at both accessory sites in A. marina may result from nearby tyrosine mutations that hydrogen bond with the Chl d formyl substitution. The latter result is consistent with evidence that the D1-side accessory chlorophyll serves as the de facto primary donor in Acaryochloris.
Continued modeling studies, in conjunction with planned photoacoustic investigations of A. marina and other oxygenic species, are anticipated to provide insight on fundamental
constraints that determine the long-wavelength (low photon-energy) limit of oxygenic photosynthesis, and thereby on extrasolar light environments where spectral signatures of
biogenic oxygen might be observed. (Figure 2A, Figure 2B) References:
[1] W.D. Swingley, et al., Niche adaptation and genome expansion in the chlorophyll d-producing cyanobacterium Acaryochloris marina, Proc. Natl. Acad. Sci. USA 105 (2008) 2005–2010.
[2] D.C. Mauzerall, Determination of oxygen emission and uptake in leaves by pulsed, time resolved photoacoustics, Plant Physiol. 94 (1990) 278–283.
[3] S.P. Mielke, N.Y. Kiang, R.E. Blankenship, M.R. Gunner, D. Mauzerall, Efficiency of photosynthesis in a Chl d-utilizing cyanobacterium is comparable to or higher than that in Chl a-utilizing
oxygenic species, Biochim. Biophys. Acta 1807 (2011) 1231–1236.
[4] S.P. Mielke, N.Y. Kiang, R.E. Blankenship, and D. Mauzerall, Wavelength-dependence of the in vivo energy-storage efficiency in the cyanobacterium, Acaryochloris marina. In preparation.
[5] Y. Song, J. Mao, M.R. Gunner, MCCE2: Improving protein pKa calculations with extensive side chain rotamer sampling, J. Comput. Chem. (2009), doi:10.1002/ jcc.21222.
[6] M. Dong, S.P. Mielke, M.R. Gunner. Comparison of A. marina and T. elongatus PSII reaction centers. In preparation.
Figure 1. Figure 1: (A) Energy-storage efficiency (ε) in A. marina whole cells as a function of pulse wavelength between 670 and 760 nm (green squares) and in S. leopoliensis at 670 nm (gray square). The fit (red curve) for λ > 730 nm assumes that the efficiency of uphill energy transfer decreases exponentially with increasing wavelength beyond that of the PSI trap. (B) Saturation profile of A. marina whole cells obtained by PA at 730 nm. The energy-storage efficiency decreases with increasing flux because of saturation of the sample obeying the cumulative one-hit Poisson function (fitted curve). Such data will help identify photosystem-specific contributions to energy storage reflected in Fig 1A.
Figure 2. Figure 2: (A) Homology model of D1 (blue) and D2 (red) protein subunits of the PSII reaction center in A. marina. The model includes the primary and accessory chlorophylls (green and orange, respectively), and the primary electron acceptor, pheophytin (yellow). Protein sequence identity with other cyanobacteria, e.g., T. elongatus, is high (~90%), leading to models with high similarity to known structures. (B) Midpoint redox potentials of the primary electron donor and acceptor within the A. marina model and T. elongatus crystal structures, calculated from MCCE titrations in which chlorophyll content was either 100% Chl a or 100% Chl d content [6].
Publications
-
Mielke, S. P., Kiang, N. Y., Blankenship, R. E., Gunner, M. R., & Mauzerall, D. (2011). Efficiency of photosynthesis in a Chl d-utilizing cyanobacterium is comparable to or higher than that in Chl a-utilizing oxygenic species. Biochimica et Biophysica Acta (BBA) – Bioenergetics, 1807(9), 1231–1236. doi:10.1016/j.bbabio.2011.06.007
- Dong, M., Mielke, S.P. & Gunner, M.R. (In Preparation). Comparison of A. marina and T. elongatus PSII reaction centers.
- Mielke, S.P. & et al. (2010). Constraining photosynthetic biosignatures: spectral photoacoustic measurements of photon energy use efficiency in the far-red/near-infrared by the chlorophyll d-utilizing cyanobacterium Acaryochloris marina. 15th International Congress on Photosynthesis. Beijing, China.
- Mielke, S.P., Kiang, N.Y., Blankenship, R.E. & Mauzerall, D. (In Preparation). Wavelength-dependence of the in vivo energy-storage efficiency in the cyanobacterium, Acaryochloris marina.
- Mielke, S.P., Kiang, N.Y., Blankenship, R.E., Gunner, M.R. & Mauzerall, D. (2010). Photosynthetic Electron-Transfer in the Cyanobacterium, Acaryochloris Marina. LPI Contributions, 1538: 5438.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Robert Blankenship
Co-Investigator
Marilyn Gunner
Co-Investigator
Nancy Kiang
Co-Investigator
David Mauzerall
Co-Investigator
-
RELATED OBJECTIVES:
Objective 5.1
Environment-dependent, molecular evolution in microorganisms
Objective 6.1
Effects of environmental changes on microbial ecosystems
Objective 6.2
Adaptation and evolution of life beyond Earth
Objective 7.2
Biosignatures to be sought in nearby planetary systems