2011 Annual Science Report
 University of Wisconsin
Reporting | SEP 2010 – AUG 2011
University of Wisconsin
Reporting | SEP 2010 – AUG 2011
Project 6B: Detection of Biosignatures in Extreme Environments and Analogs for Mars
Project Summary
We have continued to investigate the Río Tinto area an acid river in Spain analogous to the environment of early Mars. We are now using a sophisticated technique that analyses the three stable isotopes of oxygen and their relationship to each other with surprising results. Isotopic fractionation processes, change the relative proportions of the oxygen of masses 17 and 18 relative to 16 to an extent generally proportional to their mass difference, an approximately 2:1 effect for O-18 and O-17, respectively, shown by the slope of the line. Most terrestrial materials exhibit this same relationship perfectly, but oxygen in sulfate from waters and minerals in the Río Tinto area, produced by either microbial or inorganic processes, deviate from that: there is also the suggestion in preliminary data that these two processes can be differentiated from each other too. The measure of this relationship would provide a biosignature, independent of an measured oxygen isotope value of subsequent fractionation process.
Project Progress
We continued our investigation of microbial oxidation of sulfides in the Río Tinto area, southwest Spain, where long-term exploitation of pyrite (ferrous iron sulfide) has produced acid mine drainage and sulfate deposits. We have made chemical and isotopic analyses of the waters most recently. Current models for the oxidation pathways of pyrite sulfur to sulfate, and the microbial influences on those pathways are contradictory. We analyzed of Δ17O (difference from the expected 17O-18O relationship) and δ18O (a measure of 18O/16O) from various Río Tinto sources to (1) attempt to identify biomarkers in sulfate, (2) use oxygen as a source tracer to determine the oxidation pathway of pyrite sulfur to sulfate. Δ17O values (calculated as differences from the usual and expected δ 17O- δ 18O slope of 0.528).
Near the source of the Río Tinto there are quite different and clearly differentiable waters, either rich in ferric iron, Fe3+ (Fig.1) or ferrous iron, Fe2+ (Fig. 2). The differences in the iron water chemistry can be explained by microbial survival and growth strategies. This in turn leads to different oxidation pathways where (1) molecular oxygen (δ18OO2= 23.0‰) is the primary oxidant (high Fe2/Fe3+), (2) ferric iron (Fe3+) is the primary oxidant (low Fe2+/Fe3+) and water oxygen (δ18OH2O=0.0‰) is completely incorporated into sulfate.
Fig. 1 Red Río Tinto water. Water rich in ferric iron from the headwaters of the Río Tinto.
Fig. 2 Green Río Tinto water. Water rich in ferrous iron from the Zaranda Naya area of Río Tinto.
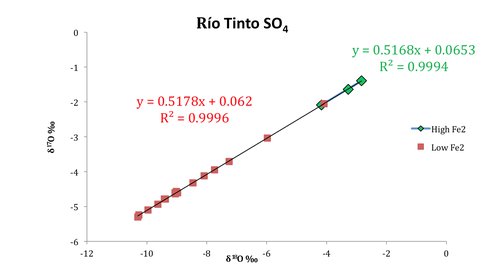
We measured a variety of different river inputs with δ18O values ranging from (+4.2 to 5.9)‰ in waters with high Fe2/Fe3+ concentrations (42%-65%), and (-2.3 to 1.1)‰ in waters with relatively low Fe2/Fe3+ concentrations (3.0%-24%), and are reproducible to 0.2‰. Although these δ18O results do indicate differences in the oxidation pathway of pyrite sulfur to sulfate, they cannot be used to quantify differences in the source of oxygen. This is because there are multiple intermediate steps during oxidation for which fractionation factors are still properly determined, and uncertainty resulting from fractionation during δ18O measurement. The Δ17O analyses of the same waters range between (+0.07 to +0.192)‰ and are reproducible to (0.007)‰. There is a strong linear relationship between δ17O and δ18O, which defines a slope or potential process specific slope of 0.5177, and an R squared value of 0.9995. This relationship has never before been measured for naturally produced sulfate via pyrite oxidation, and varies from the generally accepted relationship for terrestrial materials of 0.528 (Fig 3). This result rendered our initial strategy of using ∆17O as an oxygen source tracer infeasible, but potentially allows for the new method of measuring slope deviations specific to microbial influences.
Fig 3. Triple oxygen isotope measurements of Río Tinto waters. The line (or lines?) for oxygen in sulfate give a slope different from the expected terrestrial fractionation line (0.528). There may be a small difference also between the slopes of the lines for the two different oxidation processes, the microbially produced red, ferric waters with atmospheric oxygen as the main oxidant and those rich in ferrous iron produced abiotically by oxidation with ferric iron, using oxygen from water.
The novel method of measuring Δ17O to determine the deviation from the 0.528 line eliminates much of the uncertainty inherent to δ18O measurement. Since the Δ17O value depends only on the relationship between δ17O/δ18O, which remains constant during mass dependent fractionation, any fractionation during measurement does not affect the Δ17O value. This also means that any slope deviations caused by microbial activity will be maintained during subsequent mass dependent fractionation processes, which would change the δ18O value.
Publications
- Christensen, J., Kohl, I. & Coleman, M. (2011). Triple oxygen isotope data characterize oxidation processes that produce sulfate on Earth (and Mars?). AGU Fall meeting 2011 Abstracts.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Edward Young
Collaborator
Karen Zeigler
Collaborator
Issaku Kohl
Postdoc
Randall Mielke
Research Staff
Justin Christensen
Undergraduate Student
-
RELATED OBJECTIVES:
Objective 2.1
Mars exploration.
Objective 7.1
Biosignatures to be sought in Solar System materials