2011 Annual Science Report
 University of Wisconsin
Reporting | SEP 2010 – AUG 2011
University of Wisconsin
Reporting | SEP 2010 – AUG 2011
Project 5F: Roles of Extracellular Polysaccharides From Sulfate-Reducing Bacteria in Governing Dolomite Composition and Crystallization
Project Summary
Ca-Mg-carbonates ranging from Mg-calcite to Mg-dolomite can be synthesized in presence of extracellular polymeric substances (EPS) excreted by sulfate-reducing bacteria (SRB). The role of EPS in catalyzing dolomite formation is similar to the agar-bearing systems, because polysaccharides are the major components in EPS. Low-temperature dolomite and Ca-Mg-carbonates with continuous compositional variations between calcite and dolomite could be potential biosignatures, because they require catalysts like polysaccharides.
Project Progress
To characterize the role of extracellular polymeric substances (EPS) excreted by SRB in dolomite formation, we enriched and isolated SRB from Deep Springs Lake, California, where modern dolomite is still precipitating. EPS were extracted from the growing cultures of these microbes and further purified for synthesis experiments. Our data evidently demonstrated the catalytic effect of EPS on dolomite formation (Fig. 1). For example, the MgCO3 contents in synthetic carbonates increases with EPS concentrations in experimental solutions. With as low as 100 mg/L EPS, Ca-dolomite with 45.8 mol% MgCO3 can be synthesized (Fig. 1a). What is more interesting is that even no Mg-calcite was produced from EPS-removed dead cells-bearing solutions, while control experiments can still produce high Mg-calcite (Fig. 1b, c). Our data further deciphered the roles of SRB in dolomite formation by defining the catalytic effect of EPS.
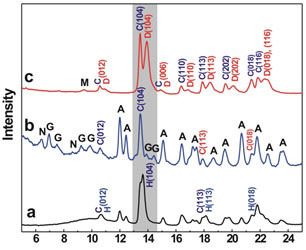
XRD patterns of synthetic carbonates from control (a), EPS-removed dead cell-bearing (b), and EPS-bearing© solutions. (a): High-Mg calcite (d104 = 2.9962 Å, 13.7 mol% of MgCO3) synthesized in control solutions (Mg:Ca = 5:1) with the presence of calcite seed crystals (0.3 g/L). (b): Aragonite and hydrated Mg-carbonates precipitated from EPS-removed dead cells-bearing solutions (Mg:Ca = 5:1) with the presence of seed crystals (0.3 g/L). (c): Ca-dolomite (d104 = 2.9323 Å, 45.8 mol% of MgCO3) synthesized in EPS-bearing solutions (Mg:Ca = 5:1) with the presence of seed crystals (0.3 g/L). A: aragonite, C: chalk calcite, D: Ca-dolomite, H: high-Mg calcite, G: giorgiosite (Mg5(CO3)4(OH)2•5H2O), N: nesquehonite (MgCO3•3H2O).
We propose that the adsorption of dissolved polysaccharides in EPS on Ca-Mg carbonate surfaces through hydrogen bonding is much stronger than that of water so that they can displace the residue hydration shells on surface Mg2+ ions, and thus promote the Mg incorporation and Ca-Mg carbonate precipitation. Published molecular dynamic calculations demonstrated a stronger surface adsorption of polysaccharides on calcite through hydrogen bonding, which support our hypothesis. Our studies can shed light on the understanding of the “dolomite problem” and the roles of microorganisms in dolomite formation. Low-temperature dolomite and Ca-Mg-carbonates with continuous compositional variations between calcite and dolomite could be potential biosignatures, because they require catalysts like polysaccharides.
Publications
-
Hong, K-S., Xu, H., Konishi, H., & Li, X. (2010). Direct Water Splitting Through Vibrating Piezoelectric Microfibers in Water. The Journal of Physical Chemistry Letters, 1(6), 997–1002. doi:10.1021/jz100027t
-
Roden, E. E., Kappler, A., Bauer, I., Jiang, J., Paul, A., Stoesser, R., … Xu, H. (2010). Extracellular electron transfer through microbial reduction of solid-phase humic substances. Nature Geosci, 3(6), 417–421. doi:10.1038/ngeo870
-
Tangalos, G. E., Beard, B. L., Johnson, C. M., Alpers, C. N., Shelobolina, E. S., Xu, H., … Roden, E. E. (2010). Microbial production of isotopically light iron(II) in a modern chemically precipitated sediment and implications for isotopic variations in ancient rocks. Geobiology, 8(3), 197–208. doi:10.1111/j.1472-4669.2010.00237.x
-
Xu, H., Chen, T., & Konishi, H. (2010). HRTEM investigation of trilling todorokite and nano-phase Mn-oxides in manganese dendrites. American Mineralogist, 95(4), 556–562. doi:10.2138/am.2010.3211
- Xu, H. (2010). Synergistic roles of microorganisms in mineral precipitates associated with deep sea methane seeps. In: Barton, L.L., Mandl, M. & Loy, A. (Eds.). Geomicrobiology: Molecular and Environmental Perspective. Springer.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Eric Roden
Co-Investigator
Hiromi Konishi
Research Staff
Evgenya Shelobolina
Research Staff
Zhizhang Shen
Graduate Student
Fangfu Zhang
Graduate Student
-
RELATED OBJECTIVES:
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems
