2011 Annual Science Report
 University of Wisconsin
Reporting | SEP 2010 – AUG 2011
University of Wisconsin
Reporting | SEP 2010 – AUG 2011
Project 5E: Disordered Dolomite Crystallization From Media Containing Agar Gel and Halophilic Bacteria
Project Summary
Ca-Mg-carbonates ranging from Mg-calcite to Mg-dolomite can be synthesized in presence of halophilic bacteria with agar media and agar media only. Extracellular polysaccharides adsorbed on surfaces can catalyze dolomite formation because of strong hydrogen bonding between the polysaccharides and the carbonate surface. This study provides insight into the effect of similar polysaccharides produced by microorganisms on dolomite formation. Low-temperature Ca-Mg-carbonate solid solution with continuous composition between calcite and dolomite could be a potential biosignature, because it requires catalysts like polysaccharides.
Project Progress
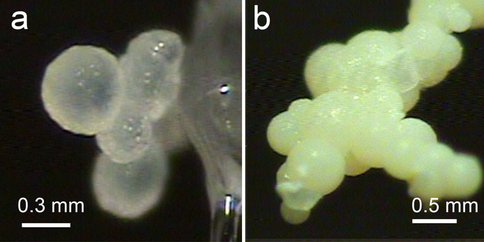
It was proposed that both the initial precipitation of Ca-dolomite and subsequent early diagenesis are strongly mediated by microbial activities. It was suggested that possible microbial activities were involved in the dolomite formation, for example, sulfate-reducing bacteria, methongens, and halophilic aerobic bacteria. However, the roles of microbes in the dolomite crystallization are still unclear. We investigated synthetic dolomites and associated phases precipitated from a media with Halomonas meridian activity at 25 °C using X-ray micro-diffraction and high resolution transmission electron microscopy (HRTEM). The results proved that synthetic dolomites are disordered dolomites instead of ordered dolomite. Synthetic dolomites occur as spherulites (100~500 um) where nano-crystals (~10 nm) aligned with their c-axes along the radius direction. The dolomite spheres have compositions ranging from 45 mole % to 55 mole % of MgCO3. Other co-existing precipitates are hydromagnesite, dypingite, and struvite. By annealing the disordered dolomite at 300 °C for 4 weeks, the Ca-Mg ordering characterized by weak superlattice reflections resulted from Ca-Mg-ordering appear. The dolomite composition and structure are very similar to abiotically synthesized disordered dolomite in presence of agar gel or dissolved agar at room temperature. Agar is water-soluble, gel-forming polysaccharide extract from agarophyte members of the Rhodophyta. Agar is usually composed of repeating D-galactose and 3,6-anhydro-L-galactopyranose units.
We propose that when dissolved in solution, polysaccharides can be adsorbed on Ca-Mg carbonate surface through hydrogen-bonding, which may lower the dielectric constant of the solution nearby the carbonate surface, enhance the desolvation and dehydration of surface Mg2+-water complexes, and catalyze Mg2+ incorporation into the precipitating Ca-Mg-carbonate. In natural environments, it is possible that polysaccharides produced by microorganisms, e.g., extracellular polysaccharides, may play a key role in promoting disordered dolomite nucleation and crystallization. This study provides insight into the effect of similar polysaccharides produced by microorganisms, i.e., extracellular polysaccharides, on dolomite formation. Low-temperature Ca-Mg-carbonate solid solutions with chemical composition between Mg-calcite and dolomite could be potential biosignatures, because they require catalysts like polysaccharides.
Optical microscope images showing aggregates of disordered dolomite (a) and hydromagnesite (b) spheres.
Publications
-
Zhang, F., Xu, H., Konishi, H., Shelobolina, E. S., & Roden, E. E. (2012). Polysaccharide-catalyzed nucleation and growth of disordered dolomite: A potential precursor of sedimentary dolomite. American Mineralogist, 97(4), 556–567. doi:10.2138/am.2012.3979
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Eric Roden
Co-Investigator
Monica Sanchez-Roman
Collaborator
Hiromi Konishi
Research Staff
Evgenya Shelobolina
Research Staff
Fangfu Zhang
Graduate Student
-
RELATED OBJECTIVES:
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems