2011 Annual Science Report
 University of Wisconsin
Reporting | SEP 2010 – AUG 2011
University of Wisconsin
Reporting | SEP 2010 – AUG 2011
Project 5C: Growth of Ca-Mg Carbonates in the Presence of Dissolved Hydrogen Sulfide - in Situ and AFM Studies
Project Summary
Dissolved hydrogen sulfide, one of the major products of bacterial sulfate reduction can promote crystallization of Ca-Mg-carbonates and dolomite. Hydrogen sulfide can be adsorbed strongly on the surfaces of Ca-Mg-carbonates, which can catalyze dehydration of surface Mg-water complexes and promote Mg incorporation into the Ca-Mg-carbonates and dolomite.
Project Progress
The “dolomite problem” has been a subject of scientific debate for decades. It has been suggested that the metabolism of anaerobic microorganism, e.g., sulfate-reducing bacteria (SRB) and methanogens, can overcome the energy barrier to dolomite crystallization. However, despite of the extensive studies on microbial dolomite formation, the specific roles of microorganism are still unclear. In the past year, we have carried out systematic studies to characterize the roles of microorganisms, especially anaerobic microorganism in sedimentary dolomite formation. In this report, we present the studies of the catalytic effect of dissolve sulfide, one of the major products of bacterial sulfate reduction (SR), and extracellular polymeric substances (EPS) excreted by SRB on dolomite crystallization.
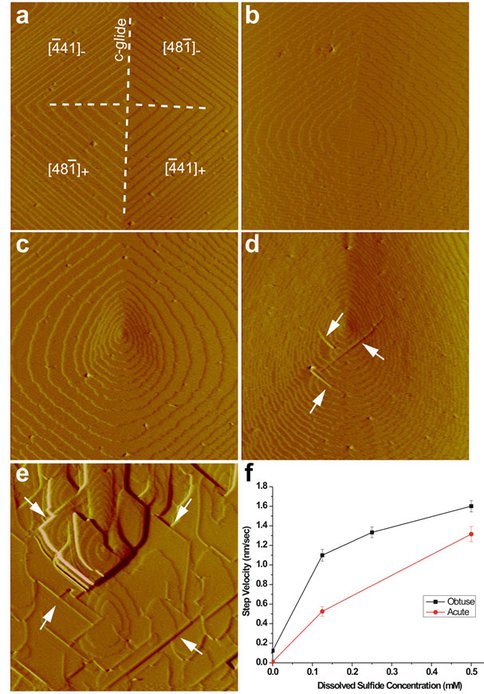
Our synthesis experiments clearly demonstrated that even millmolar level of dissolved sulfide can catalyze disordered dolomite precipitation. We also collaborated with Professor H. Henry Teng of George Washington University on in-situ atomic force microscope (AFM) observations of the nucleation and growth behavior of Ca-Mg carbonates in contacting with dissolved sulfide-bearing solutions (Fig. 1). It is shown that the presence of Mg2+ in solution has a strong inhibitory effect on both the nucleation (generation of new steps from the screw dislocation and growth (Fig. 1b). However, with the presence of dissolved sulfide, both the nucleation and growth of the hillock are enhanced, and the curved steps generated a teardrop morphology (Fig. 1c). With the continuing flow of the sulfide-bearing growth solution, some straight edges were reestablished. Most of these new edges were parallel to the calcite straight steps (Fig. 1, d and e). Newly produced steps were ‘bunched’ onto the straight edges to form 'cliffs’ which block the further growth of new steps (Fig. 1, d and e). We hypothesize the produce of these straight edges and cliffs are due to their low free energy. Due to the incorporation of Mg2+ into the growing crystal, the free energy of curved steps may become higher than that of straight ones, which therefore resulted in the appearance of the later. Our in-situ AFM studies evidently demonstrated dissolved sulfide can diminish the inhibitory effect of Mg2+ on Ca-Mg carbonate crystallization.
AFM images are 4 um by 4 um. (a): In the absence of Mg and dissolved sulfide, calcite growth hillocks develop by the propagation of straight steps that lack visible roughening. New steps are generated from the screw dislocation in the middle. The c-glide plane bisects two types of symmetrically equivalent step edges which define the positive and negative step directions to give four hillock flanks. (b): With 1 mM Mg (Mg:Ca = 5:1), step edges, particularly on positive flanks, became roughened and both the nucleation (generation of new steps from the dislocation) and growth were strongly retarded. (c): With the flow of the growth solution containing 0.13 mM dissolved sulfide and 1 mM Mg, step edges became roughened and both the negative and positive flanks grew fast which resulted in a teardrop morphology. (d) and (e): With the continuing flow of this growth solution, some straight edges were reestablished. Most of these new edges were parallel to the calcite straight steps (arrows). Newly produced steps were ‘bunched’ onto the straight edges to form ‘cliffs’ which block the further growth of new steps. (f): Kinetic measurements of step propagation rate versus dissolved sulfide concentration.
Publications
- Shen, Z., Liu, Y., Zhang, F., Kemp, J., Szlufarska, I. & Xu, H. (2011). Modeling the effect of dissolved hydrogen sulfide on Mg2+-water complex on dolomite {104} surface. Annual Meeting of the Geological Society of America. Minneapolis.
- Zhang, F., Xu, H., Konishi, H., Yan, C., Teng, H. & Roden, E.E. (2011). Aqueous sulfide-catalyzed nucleation and growth of disordered dolomite at room temperature. Annual Meeting of the Geological Society of America. Minneapolis.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Eric Roden
Co-Investigator
Henry Teng
Collaborator
Hiromi Konishi
Research Staff
Fangfu Zhang
Graduate Student
Joshua Kemp
Undergraduate Student
-
RELATED OBJECTIVES:
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems