2011 Annual Science Report
 University of Wisconsin
Reporting | SEP 2010 – AUG 2011
University of Wisconsin
Reporting | SEP 2010 – AUG 2011
Project 5B: Magnesium Isotope Fractionation Between Calcite and Aqueous Mg
Project Summary
The isotopic composition of Mg in carbonate is of interest to studies on paleo environments because of the potential for constraining temperatures, vital effects, and Mg fluxes associated with precipitation of carbonate minerals. Indeed, Mg isotope fractionation during inorganic carbonate precipitation is important because this serves as the baseline for interpreting the Mg isotope variations and inferred fluid isotope compositions for both abiogenic and biogenic carbonate. We report the results of Mg-bearing calcite synthesis studies used to constrain the fractionation of Mg isotopes during calcite precipitation. The results provide the baseline data needed to interpret Mg-bearing carbonates from the early Earth, as well as carbonates that are likely to be found on Mars.
Project Progress
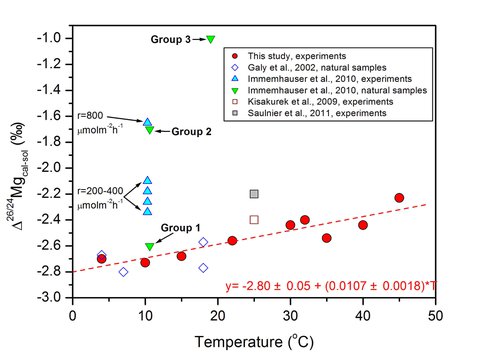
To determine the baseline Mg isotope fractionation factor for abiogenic carbonate precipitation, thirty free-drift synthesis experiments of carbonate were carried out at temperatures between 4 °C and 45 °C, using solutions with Mg:Ca molar ratio between 3:1 and 13:1, buffered at PCO2 between 0.038% and 3%. Pure calcite seed crystals were used to promote heterogeneous growth of Mg-bearing calcite solid form solution and to reduce kinetic isotope effects associated with nucleation and rapid crystallization. Under such conditions, calcite overgrowths (confirmed by XRD) that contained 1.28-14.9 mole percent Mg precipitated onto the seed crystals over 1 to 58 days. The Mg isotope composition of Mg-bearing calcite and Mg-Ca solutions were measured to a precision of ±0.15‰ (2 standard deviation in 26Mg/24Mg) after careful purification of Mg from these Ca-rich materials using multiple ion-exchange separation techniques. The measured Mg isotope fractionation factors between Mg-calcite and solution show a systematic temperature dependence, changing from -2.26 ‰ at 45 °C to -2.76 ‰ at 4 °C in 26Mg/24Mg (Figure 1).
Comparison of experimental determined Δ26Mgcal-sol values with fractionation
factors reported by other experimental and field studies. Errors of these data are typically
within 0.1-0.2 ‰. The precipitation rates® for synthesized Mg-bearing calcite reported
by Immenhauser et al. (2010) are denoted. Immenhauser et al. (2010) reported three
groups of samples from natural environments, among which, Group 1 are slowly
precipitated speleothem samples, Group 2 are surficial calcrete of speleothem, and Group
3 are freshly precipitated calcite collected using watch glass. Precipitation rates are
suggested to increase from Group 1 to Group3.
The fractionation factors are not correlated with experimental settings, Mg content of the Mg-calcite overgrowth, PCO2, or the composition of the Mg-Ca solution. This study proposes a temperature-dependent function for Mg isotope fractionation during precipitation of inorganic Mg-bearing calcite: Δ26/24Mgcal-sol = -2.82(±0.11) + 0.0119(±0.0038)×T, where T is temperature in Celsius.
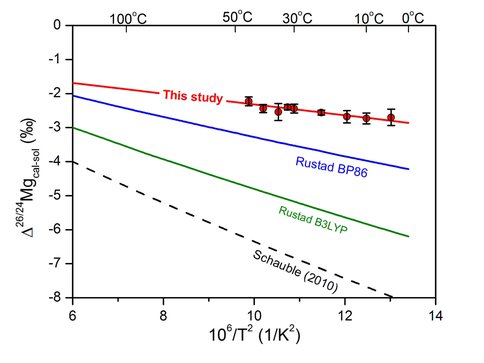
Speleothem studies define similar temperature dependence for Mg isotope fractionation factor between Mg-calcite and companion aqueous Mg drip waters assuming an average speleothem formation occurred at the average annual cave temperature (Figure 1). In contrast to the excellent agreement between natural samples and our experiments, calculated calcite-solution Mg isotope fractionation factors do not agree, where calculations predict a larger magnitude fractionation as compared to experimental results (Figure 2).
Comparison of theoretically predicted Mg isotope fractionation factors
between calcite and solution (Δ26/24Mgcal-sol) with data determined from this
experimental study. The fractionation factor for calcite calculated by Rustad et al. (2010) was done using two different models (BP86 and B3LYP). The curve labeled Schauble uses the aqueous Mg calculation of Schauble (2010) and the BP86 calcite formulation for calcite of Rustad et al. (2010; the B3LYP would plot at more negative values). The curves labeled Rustad use the BP86 and B3LYP formulations for both calcite and aqueous Mg reported by Rustad et al. (2010).
It is uncertain if these differences reflect scaling problems between the calculated fluid and solid beta values (e.g. Beard et al., 2010), inaccuracies associated with difficulties in modeling of the hydration sphere of the aqueous cation (Rustad et al., 2010), or if the assumed crystallographic model for calcite with an infinitesimal amount of Mg does not accurately reflect natural lattice settings. Alternatively, this discrepancy may reflect experimental kinetic isotope effects associated with rapid precipitation. However, because our experimental study employed calcite seed crystals to decrease precipitation rates by minimizing possible crystallization nucleation problems and the fact that our experiments are in excellent agreement with natural speleothem studies, we believe that the experimental results accurately reflect Mg isotope fractionation associated with abiogenic precipitation of Mg-bearing calcite.
Publications
-
Li, W., Chakraborty, S., Beard, B. L., Romanek, C. S., & Johnson, C. M. (2012). Magnesium isotope fractionation during precipitation of inorganic calcite under laboratory conditions. Earth and Planetary Science Letters, 333-334, 304–316. doi:10.1016/j.epsl.2012.04.010
- Temperature-dependent Mg isotope fractionation during precipitation of inorganic calcite under laboratory conditions.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Clark Johnson
Co-Investigator
Christopher Romanek
Co-Investigator
Suvankar Chakraborty
Postdoc
Weiqiang Li
Postdoc
-
RELATED OBJECTIVES:
Objective 4.1
Earth's early biosphere.
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems