2011 Annual Science Report
 University of Wisconsin
Reporting | SEP 2010 – AUG 2011
University of Wisconsin
Reporting | SEP 2010 – AUG 2011
Project 4C: Iron Isotope Geochemistry in Biogenic Magnetite-Bearing Lake Sediments
Project Summary
The production of magnetite as a byproduct of dissimilatory microbial iron oxide reduction (DIR) has been hypothesized to be an important pathway in the early diagenesis of chemically-precipitated sediments on early Earth, leading ultimately to the preservation of large quantities of magnetite in banded iron formations (BIFs). A significant fraction of the magnetite (and other Fe-bearing minerals such as siderite and pyrite) in BIFs is isotopically light, likely due to Fe isotope fractionation between biogenic Fe(II) and residual Fe(III) oxides. Only one modern environmental setting has reported possible in situ magnetite formation resulting from DIR: the Bay of Vidy in Lake Geneva, Switzerland. Previous work has characterized a widespread magnetic susceptibility anomaly in the Bay of Vidy sediments stemming from an influx of amorphous Fe(III) oxide from a nearby sewage treatment plant, and determined the presence of fine-grained magnetite apparently produced via DIR. In this study, we examined the Fe isotope composition of distinct pools of solid-phase Fe contained in sediments from the Bay of Vidy. Significant Fe(III) reduction has taken place, resulting in the reduction of nearly all reactive (non-silicate) Fe. Very little Fe isotope variation was observed within sediment Fe pools, including magnetite. The lack of sediment heterogeneity, along with the highly reduced nature of the sediments, suggests that DIR has carried through to completion in this deposit. The absence of spatial Fe redox gradients accompanying complete Fe(III) reduction has prevented the segregation of Fe isotopes during microbial reduction. This case study provides a basis for interpreting instances in the rock record where DIR was active but no Fe isotope fractionation was preserved.
Project Progress
Magnetite, Fe3O4, is a ferrimagnetic mineral common in Precambrian rocks, specifically banded iron formations (BIFs). Based on geochemical and microbiological evidence it has been hypothesized that dissimilatory microbial iron oxide reduction (DIR) may have been responsible for the formation of magnetite in BIFs (Cloud, 1974; Johnson et al., 2008a; Konhauser et al., 2005; Nealson and Myers, 1990; Vargas et al., 1998; Walker, 1984). DIR is a microbial metabolism involving the bacterial the reduction of Fe(III) coupled to the oxidation of H2 or organic compounds (Lovley et al., 2004). Magnetite is a common product of microbial reduction of poorly crystalline Fe(III) oxides in laboratory systems (Borch et al., 2007; Lovley et al., 1987; Perez-Gonzalez et al., 2010; Roden and Lovley, 1993; Roh et al., 2003; Salas et al., 2010). Although poorly crystalline iron oxides are ubiquitous in nature, the formation of significant quantities of magnetite associated with DIR has only been reported for one modern location on earth: the Bay of Vidy in Lake Geneva, Switzerland (Gibbs-Eggar et al., 1999). Bay of Vidy is a unique environment where an influx of amorphous Fe(III) oxides to the lake bottom has taken place through the outflow of a local municipal sewage treatment plant. Previous investigations in Bay of Vidy sediments have identified significant quantities of fine-grained magnetite, chemically identical to those formed as a byproduct of microbial Fe(III) oxide reduction in laboratory cultures, which demonstrate temporal correlations to the use of ferric chloride for wastewater treatment (Gibbs-Eggar et al., 1999). No additional work, however, has been conducted on the magnetite in these sediments and the potential use of these deposits as proxy for DIR-driven microbial magnetite formation on ancient earth.
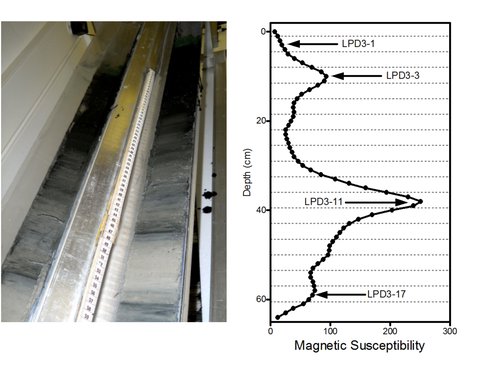
This study generated geochemical and isotopic data from magnetite-bearing sediments collected from the Bay of Vidy in September 2010. Measurements of magnetic susceptibility
Ph.D. student Liz Percak-Dennett deploying a sediment corer at the Bay of Vidy in Lake Geneva with Dr. Jean-Luc Loizeau of the Institut F.-A. Forel, Université de Genève (left); Liz capping a core (middle); and processing the core material in an anaerobic glove bag (right).
were conducted to identify sediment depth intervals for mineralogical, geochemical, and Fe isotopic analyses. We focused on four depth intervals (1-4.5, 8-11.5, 36-39.5, and 57-60.5 cm), spanning a range of magnetic susceptibility values.
Photo of Lake Geneva sediment core during measurement of magnetic susceptibility (left) and profiles of sediment magnetic susceptibility in core LPD3 (right). Arrows indicate depths that were samples for Fe geochemical and isotopic analysis.
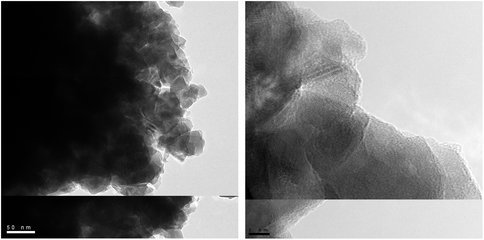
Wet chemical extractions indicated that virtually all of Fe(III) oxides entering the sediment are reduced within the upper few cm of sediment. Most of the reduced iron, Fe(II), was present as nonsulfide-associated amorphous phases, with relatively small (less than one-third of total reactive, non-silicate Fe) amounts of acid volatile sulfide (iron monosulfide) and pyrite. The relatively small amounts of Fe-S phases in Bay of Vidy sediment (e.g. compared to typical modern marine sediments) can be explained by the low abundance of sulfate in Lake Geneva waters (few hundred μM) relative to seawater (28 mM). Magnetite particles (obtained via magnetic separation; see Figure 3) accounted for 1-10% of total reactive Fe.
TEM images of magnetite crystallites in Bay of Vidy sediments, recovered with a hand magnet from the interval of maximum magnetic susceptibility (36-39.5 cm depth) in core LPD3.
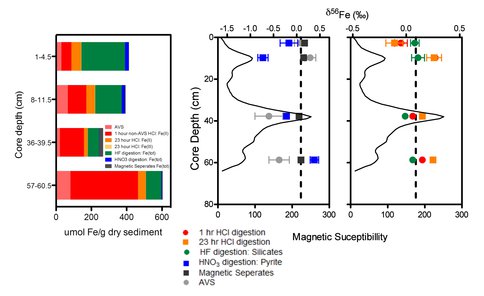
Iron isotope measurements revealed a narrow range (less than one ‰) of δ56Fe values.
In particular, δ56Fe values for the magnetic separates were nearly identical to the mean bulk (total) sediment value of 0.10 ± 0.05 ‰.
Left: Pools of Fe recovered by sequential extraction of sediment from different depths in core LPD3. Right: delta56Fe values for extracted Fe pools; magnetic susceptibility is plotted as a black line on the lower X-axis, and delta56Fe data are plotted on the upper X-axis. Dashed lines represent average delta56Fe measured for bulk sediment digestions.
The same was true for the large pool of nonsulfide-associated Fe(II). Only Fe associated with reduced S phases showed significant fractionation, although the mean values for these phases (-0.19 ± 0.45 for iron monosulfide, -0.15 ± 0.41 for pyrite) were close to the average bulk value. There was no obvious correlation between δ56Fe values and magnetite susceptibility or magnetite abundance, which makes sense given that magnetite accounted for only a minor fraction of reactive Fe.
The results of this study yield new insight into Fe isotope behavior in sediments undergoing extensive microbial Fe(III) reduction. In particular, the complete conversion of reactive Fe(III) to Fe(II) has apparently prevented significant separation of light and heavy Fe isotopes, which is known to take place during partial reduction of Fe(III) oxide phases in sediment (Severmann et al., 2006; Tangalos et al., 2010). Burial diagenesis of sediments in which only partial Fe(III) reduction takes place can lead to preservation of Fe isotope signatures of microbial Fe(III) oxide reduction, specifically the sequestration of isotopically-light Fe in minerals such as magnetite and siderite in Fe-rich rocks (e.g. BIFs) (Johnson et al., 2008a; Johnson et al., 2008b). In contrast, if Bay of Vidy sediments were to undergo diagenesis as they exist now, no heterogeneity or low-δ56Fe values would be preserved in the rock record. This illustrates the inability of iron isotope analysis to accurately portray microbial Fe(III) reduction in systems that have undergone near complete reduction, and brings to attention the value of rocks in the geologic record that contain minerals of biological origin, but which do not show variations in δ56Fe values.
Publications
-
Percak-Dennett, E. M., Loizeau, J-L., Beard, B. L., Johnson, C. M., & Roden, E. E. (2013). Iron isotope geochemistry of biogenic magnetite-bearing sediments from the Bay of Vidy, Lake Geneva. Chemical Geology, 360-361, 32–40. doi:10.1016/j.chemgeo.2013.10.008
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Brian Beard
Co-Investigator
Clark Johnson
Co-Investigator
Elizabeth Percak-Dennett
Doctoral Student
-
RELATED OBJECTIVES:
Objective 4.1
Earth's early biosphere.
Objective 7.2
Biosignatures to be sought in nearby planetary systems