2011 Annual Science Report
 University of Wisconsin
Reporting | SEP 2010 – AUG 2011
University of Wisconsin
Reporting | SEP 2010 – AUG 2011
Project 3C: Integration of Multiple Isotope Proxies to Study the Pre-GOE Oxygenation of the Earth
Project Summary
The period 2.7 to 2.5 b.y. ago, the period leading you to the Great Oxidation Event (GOE), is becoming increasingly recognized as a time of major environmental change. A holistic understanding of the changes that occurred in microbial ecology, and their effects of the environment, are only possible by integrating multiple geochemical proxies. By simultaneously looking at C, O, S, Fe, Mo, and Sr isotopes, we develop a picture of extensive oxygenic photosynthesis, but approximate balance with reduced resevoirs such as reduced Fe and reduced volcanic gases, such that free oxygen did not yet become abundant on the planet. Although many workers have questioned a rise in oxygenic photosynthesis significantly before the GOE, these new data clearly indicate that this metabolism was widespread at least 400 m.y. before the GOE.
Project Progress
Marine sedimentary rocks are logical targets for extracting geochemical proxies for the ancient surface environments of the Earth. Many workers would agree that shallow-water, Ca-Mg carbonates may be suitable for understanding processes that occurred in the photic zone of Archean and Proterozoic oceans. For Ca-Mg carbonates that are carefully selected to avoid alteration and diagenetic effects, their isotopic compositions are likely to reflect those of the ancient shallow oceans. This in turn allows inferences to be made of some, but not all, components of the biogeochemical cycles that operated on the surface of the Earth in the past. Because, however, Ca-Mg carbonates have low Fe contents, they do not directly record microbial metabolisms that are associated with the Fe cycle; insights into the Fe cycle must come from direct study of Fe-rich rocks such as banded iron formations (BIFs).
In this study, C, O, S, Fe, Mo, and Sr isotopes from 2.6-2.5 Ga Campbellrand carbonate platform (South Africa) were combined into an integrated model for the Neoarchean oceans. The ancient marine record is best studied by dividing the record into Fe-rich and Fe-poor lithologies. The Fe-poor record recorded in Ca-Mg carbonates probably reflects removal of Fe from the shallow oceans via oxidation of hydrothermally sourced Fe(II)aq from reduced, deeper portions of the oceans, followed by precipitation as ferric oxides/hydroxides that settled back to the deep ocean. This follows common models of the Archean oceans that have deep, Fe(II)-rich zones (e.g., Holland, 1984). Although it has been proposed that oxidation of Fe(II)aq could have occurred in the absence of biology by UV-photo oxidation (e.g., Braterman and Cairnssmith 1987), this mechanism is considered less likely based on experiments using seawater analogs (Konhauser et al. 2007), leaving us with photosynthetic pathways for oxidation. Oxidation may have occurred by anaerobic photosynthetic Fe(II) oxidation (e.g., Canfield 2005; Olson 2006; Widdel et al. 1993), or oxygenic photosynthesis; a preponderance of the data suggests that oxygenic photosynthesis developed in the Paleoarchean based on morphological, phylogenetic, molecular biomarker, and C isotope data (see review by Farquhar et al. 2011). It is anticipated that extensive Fe(II) oxidation would precede rise of free oxygen in the atmosphere, since only after all sinks for oxygen were exhausted could a significant rise in O2 occur (e.g., Claire et al. 2006; Goldblatt et al. 2006; Kump and Barley 2007). Given the great abundance of Fe(II) in hydrothermal fluids and igneous and metamorphic rocks, Fe(II) was likely a very important sink for oxygen during the Archean.
The above discussion suggests that marine sedimentary rocks that reflect accumulation of Fe, such as Fe-rich shales and iron formations, reflect a complementary side of surface biogeochemical pathways that is not represented by low-Fe, Ca-Mg carbonates. The C, O, Fe, and Sr isotope compositions of iron formation carbonates of the Campbellrand carbonate platform indicate that these minerals did not form in equilibrium with seawater. Given the large ferric oxide/hydroxide and organic carbon fluxes to the seafloor that would accompany oxygenic photosynthesis in the Neoarchean, conditions would have been ideal to support dissimilatory iron reduction (DIR). DIR is phylogenetically diverse, deeply rooted in the Bacteria and Archaea, including hyperthermophiles, sulfate reducers, nitrate reducers, and methanogens, involving a wide variety of electron donors (e.g., Lovley et al. 2004), and is considered to be one of the earliest microbial metabolisms on Earth (Lovley 2004; Vargas et al. 1998). Although bacterial sulfate reduction (BSR) has figured prominently in the geochemical literature for several decades, the importance of DIR as an ancient biogeochemical process is only now being widely recognized in this literature. Nevertheless, it is important to note that Walker (1984) proposed that Fe(III) was likely the most important electron acceptor in environments of BIF deposition. It is therefore concluded that the isotopic compositions of the Neoarchean and Paleoproterozoic iron formations discussed here were largely controlled by microbial processes in the soft sediment prior to lithification, and therefore cannot be used as a paleo proxy for deep ocean water.
When the broad temporal variations in isotopic compositions of marine sedimentary rocks of Archean and Proterozoic age are simultaneously viewed from the perspective of paleoenvironmental proxies and microbial cycling, many previously puzzling data, as well as new data, begin to fall into place. For example, Johnson et al. (2008) proposed that expansion of DIR as an important microbial metabolism was most likely when marine sulfate contents were low, which would produce low levels of sulfide production by BSR, allowing reactive Fe(III) oxides/hydroxides to be utilized by DIR. In addition to Fe isotopes, C, O, S, and Sr isotope variations in detailed studies of Neoarchean and Paleoproterozoic basins (Figure 1) support such a model. The results of this study demonstrate that the question “do Archean and Proterozoic isotopic records reflect paleo-ocean proxies or microbial cycling?” does not have a single answer. Full understanding of the environmental and biological information contained in the isotopic compositions of ancient rocks requires looking at many isotopic systems at once, in addition, of course, to a firm geologic understanding of the samples.
References Cited:
Beukes NJ, Klein C (1990) Geochemistry and Sedimentology of a Facies Transition – from Microbanded to Granular Iron-Formation – in the Early Proterozoic Transvaal Supergroup, South-Africa. Precamb Res 47(1-2):99-139
Beukes NJ, Klein C, Kaufman AJ, Hayes JM (1990) Carbonate Petrography, Kerogen Distribution, and Carbon and Oxygen Isotope Variations in an Early Proterozoic Transition from Limestone to Iron-Formation Deposition, Transvaal Supergroup, South-Africa. Econ Geol 85(4):663-690
Braterman PS, Cairnssmith AG (1987) Photoprecipitation and the Banded Iron-Formations – Some Quantitative Aspects. Origins Life Evol Biosphere 17(3-4):221-228
Canfield DE (2005) The early history of atmospheric oxygen: Homage to Robert Garrels. Ann Rev Earth Planet Sci 33:1-36
Claire MW, Catling DC, Zahnle KJ (2006) Biogeochemical modeling of the rise in atmospheric oxygen. Geobiol 4:1-31
Czaja AD, Johnson CM, Beard BL, Roden EE, Voegelin AR, Nagler TF, Beukes NJ (2011) Evidence for free oxygen in the Neoarchean ocean based on coupled iron-milybdenum isotope fractionation. Geochim Cosmochim Acta submitted
Farquhar J, Zerkle AL, Bekker A (2011) Geological constraints on the origin of oxygenic photosynthesis. Photosyn Res 107(1):11-36
Fischer WW, Schroeder S, Lacassie JP, Beukes NJ, Goldberg T, Strauss H, Horstmann UE, Schrag DP, Knoll AH (2009) Isotopic constraints on the Late Archean carbon cycle from the Transvaal Supergroup along the western margin of the Kaapvaal Craton, South Africa. Precamb Res 169:15-27
Goldblatt C, Lenton TM, Watson AJ (2006) Bistability of atmospheric oxygen and the Great Oxidation. Nature 443:683-686
Heimann A, Johnson CM, Beard BL, Valley JW, Roden EE, Spicuzza MJ, Beukes NJ (2010) Fe, C, and O isotope compositions of banded iron formation carbonates demonstrate a major role for dissimilatory iron reduction in similar to 2.5 Ga marine environments. Earth Planet Sci Lett 294(1-2):8-18
Johnson CM, Beard BL, Beukes NJ, Klein C, O’Leary JM (2003) Ancient geochemical cycling in the Earth as inferred from Fe isotope studies of banded iron formations from the Transvaal Craton. Contrib Mineral Petrol 144(5):523-547
Johnson CM, Beard BL, Roden EE (2008) The iron isotope fingerprints of redox and biogeochemical cycling in the modern and ancient Earth. Ann Rev Earth Planet Sci 36:457-493
Kamber BS, Webb GE (2001) The geochemistry of late Archaean microbial carbonate: Implications for ocean chemistry and continental erosion history. Geochim Cosmochim Acta 65(15):2509-2525
Kamber BS, Whitehouse MJ (2007) Micro-scale sulphur isotope evidence for sulphur cycling in the late Archean shallow ocean. Geobiol 5(1):5-17
Kaufman AJ (1996) Geochemical and mineralogic effects of contact metamorphism on banded iron-formation: an example from the Transvaal Basin, South Africa. Precamb Res 79:171-194
Kaufman AJ, Johnston DT, Farquhar J, Masterson AL, Lyons TW, Bates S, Anbar AD, Arnold GL, Garvin J, Buick R (2007) Late Archean biospheric oxygenation and atmospheric evolution. Science 317(5846):1900-1903
Kendall B, Reinhard CT, Lyons T, Kaufman AJ, Poulton SW, Anbar AD (2010) Pervasive oxygenation along late Archaean ocean margins. Nature Geoscience 3(9):647-652
Knoll AH, Beukes NJ (2009) Introduction: Initial investigations of a Neoarchean shelf margin-basin transition (Transvaal Supergroup, South Africa). Precamb Res 169(1-4):1-14
Konhauser KO, Amskold L, Lalonde SV, Posth NR, Kappler A, Anbar A (2007) Decoupling photochemical Fe(II) oxidation from shallow-water BIF deposition. Earth Planet Sci Lett 258:87-100
Kump LR, Barley ME (2007) Increased subaerial volcanism and the rise of atmospheric oxygen 2.5 billion years ago. Nature 448(7157):1033-1036
Lovley DR (2004) Potential role of dissimilatory iron reduction in the early evolution of microbial respiration. In: Seckbach J (ed) Origins, evolution, and biodiversity of microbial life, vol. Kluwer, Netherlands, pp 301-313
Lovley DR, Holmes DE, Nevin KP (2004) Dissimilatory Fe(III) and Mn(IV) reduction. Advances in Microbial Physiology 49:219-286
Ludois JM, Johnson CM, Beard BL, Beukes NJ, Heimann A (2011) Strontium isotopes in Banded Iron Formation carbonates demonstrate disequilibrium with ancient seawater. Precamb Res submitted
Olson JM (2006) Photosynthesis in the Archean Era. Photosyn Res 88:109-117
Ono SH, Kaufman AJ, Farquhar J, Sumner DY, Beukes NJ (2009) Lithofacies control on multiple-sulfur isotope records and Neoarchean sulfur cycles. Precamb Res 169(1-4):58-67
Vargas M, Kashefi K, Blunt-Harris EL, Lovley DR (1998) Microbiological evidence for Fe(III) reduction on early Earth. Nature 395:65-67
Voegelin AR, Nagler TF, Beukes NJ, Lacassie JP (2010) Molybdenum isotopes in late Archean carbonate rocks: Implications for early Earth oxygenation. Precamb Res 182(1-2):70-82
Von Blanckenburg F, Mamberti M, Schoenberg R, Kamber BS, Webb GE (2008) The iron isotope composition of microbial carbonate. Chem Geol 249:113-128
Walker JCG (1984) Suboxic diagenesis in Banded Iron Formations. Nature 309(5966):340-342
Widdel F, Schnell S, Heising S, Ehrenreich A, Assmus B, Schink B (1993) Ferrous Iron Oxidation by Anoxygenic Phototrophic Bacteria. Nature 362(6423):834-836
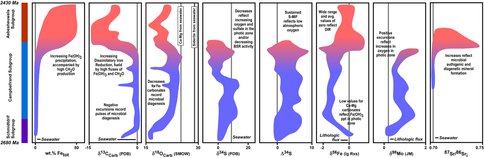
Figure 1. Summary of variations in chemical and isotopic compositions of the Neoarchean Campbellrand-Malmani carbonate platform of the Transvaal basin, South Africa. Fields reflect correlation of the base of the Kuruman iron formation and Klein Naute Formation and Tsineng member of the Gamohaan Formation. Due to large variations in stratigraphic thicknesses, chemical and isotopic variations should be considered schematic and not strictly tied to specific core sections, although the trends illustrated closely follow the data as related to the Schmidtdrift-Campbellrand-Asbesheuwels subgroups. Color variations from blue to red reflect the broad transitions from Ca-Mg carbonates to iron formation deposition. Data sources for platform samples: Data from Beukes et al. (1990; cores AD-5, WB-98, DI-1), Beukes and Klein (1990; core CN-109), Kaufman (1996; cores AD-5, CN-109, WB-98, and DI-1), Kamber and Webb (2001; unamed core S of Kuruman), Johnson et al. (2003; cores AD-5, CN-109), Kaufman et al. (2007; core AD-5), Kamber and Whitehouse (2007; unamed core S of Kuruman), von Blanckenburg et al. (2008; unamed core S of Kuruman), Fischer et al. (2009; core BH 1-SACHA), Heimann et al. (2010; cores AD-5, WB-98, DI-1), and Ludois et al. (2011; cores AD-5, WB-98, DI-1). Data sources for slope samples (Agouron Institute cores GKF01 and GKP01): Ono et al. (2009), Fischer et al. (2009), Kendall et al. (2010), Voegelin et al. (2010), and Czaja et al. (2011).
Publications
-
Czaja, A. D., Johnson, C. M., Roden, E. E., Beard, B. L., Voegelin, A. R., Nägler, T. F., … Wille, M. (2012). Evidence for free oxygen in the Neoarchean ocean based on coupled iron–molybdenum isotope fractionation. Geochimica et Cosmochimica Acta, 86, 118–137. doi:10.1016/j.gca.2012.03.007
-
Czaja, A. D., Johnson, C. M., Yamaguchi, K. E., & Beard, B. L. (2012). Comment on “Abiotic Pyrite Formation Produces a Large Fe Isotope Fractionation”. Science, 335(6068), 538–538. doi:10.1126/science.1211804
- Johnson, C. (2011, Submitted). Archean Isotopic Records: Paleo-Ocean Proxies or Microbial Cycling? Geol. Soc. Amer. Bull.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Brian Beard
Co-Investigator
Nicolas Beukes
Collaborator
Thomas Nägler
Collaborator
Andrea Voegelin
Collaborator
Andrew Czaja
Postdoc
Jim Ludois
Graduate Student
-
RELATED OBJECTIVES:
Objective 4.1
Earth's early biosphere.
Objective 5.3
Biochemical adaptation to extreme environments
Objective 6.1
Effects of environmental changes on microbial ecosystems
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems