2011 Annual Science Report
 University of Wisconsin
Reporting | SEP 2010 – AUG 2011
University of Wisconsin
Reporting | SEP 2010 – AUG 2011
Project 2C: Role of Extracellular Polymeric Substances (EPS) and Bacteria Cell Wall Structure in Shielding Against Specific Mineral Toxicity - Implications for Cell Surface Evolution
Project Summary
Our interdisciplinary project examined the hypotheses that (1) bacterial cell membranes are ruptured in contact with specific mineral surfaces, (2) biofilm-forming extra-cellular polymeric substances (EPS) may have evolved to shield against membrane rupture (cell lysis), (3) differences in cell-wall structure of Gram-negative and Gram-positive bacteria may influence the susceptibility of cells to toxic minerals and (4) mineral toxicity depends on its surface chemistry and nanoparticle size.
Previously, we have examined Gram-negative P. aeruginosa strains, wild-type (PAO1) that is capable of generating copious amount of EPS and producing biofilms, as well as the knock-out mutant (Δ-psl) that is defective in its ability to form EPS and biofilms. In the 2010-2011 year of funding, we have expanded our study to include Gram-positive B. subtilis strains, biofilm-producing wild-type NCIB3610 and biofilm-defective mutant yhxBΔ. We confirmed the hypotheses (1), (2) and (4) for both Gram-negative and Gram-positive bacteria, with toxicity increasing as amorphous β-TiO2 < γ-Al2O3. We also confirmed hypothesis (3) that Gram-positive bacteria are less susceptible to mineral toxicity than Gram-negative because of the most robust cell-wall structure of the former. Finally, we have shown that the mechanisms of toxicity depends on mineral surface charge for initial adhesion of nanoparticles to the cell surface, nanoparticle size which determines whether the particles can enter the intracellular space (e.g., for γ-Al2O3), the presence of surface free radicals (e.g., β-TiO2 ) which would have been generated by UV-radiation and meteorite impacts on early Earth, Mars, and other worlds.
By understanding the mechanisms for membranolysis, especially under the extreme conditions of high radiation and heavy impacts during early planetary history, the project addresses the NASA Astrobiology Institute’s (NAI) Roadmap goals of understanding the origins of cellularity, the evolution of mechanisms for survival at environmental limits, and preservation of biosignatures, and NASA’s Strategic Goal of advancing scientific knowledge of the origin and evolution of the Earth’s biosphere and the potential for life elsewhere.
Project Progress
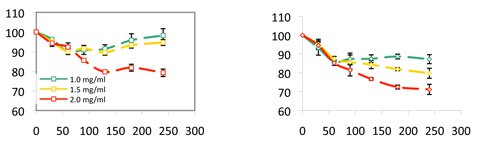
In the present year, we initiated and completed a study to determine if the EPS of Gram-positive bacteria acts to shield them against the cytotoxic effects of different oxides (anatase and alumina). We used wild-type , biofilm-producing wild-type NCIB3610 and biofilm-defective mutant yhxBΔ (Figure 1). We confirmed that the presence of EPS does protect Gram-positive bacteria from toxic oxides (Figure 2). We have previously shown the same result for Gram-negative strains. Thus, our hypothesis that EPS may have evolved early in the evolution of life seems to hold across the two bacterial types.
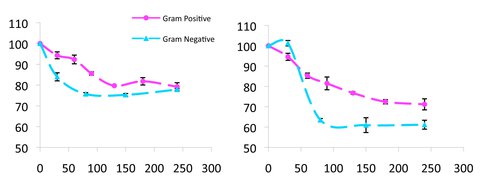
We also conducted fresh, parallel experiments on Gram-negtive bacteria, the P. aeruginosa strains, wild-type (PAO1) and the knock-out mutant Δ-psl for consistency of these results with the Gram-positive bacteria. We showed that the Gram-positive cel –wall which has a thick outer layer of peptidoglycan provides additional protection against the toxicity of oxides (Figure 3).
Finally, we confirmed that the toxicity of oxides depends on the surface charge of each oxide with anatase being less toxic than alumina. Thus, not all minerals can be generalized as being either “benign” or “toxic” towards the early evolution of life.
Figure 1. Relative viability (%) (y-axis) in the presence of anatase at different loadings compared to anatase-free controls as a function of time (min) (x-axis) for Gram-positive washed wild-type NCIB3610 (left) and washed mutant yhxBΔ (right) strains. Note reduced cell viability of EPS-defective mutants compared to the wild-type that make EPS and biofilms, indicating that EPS provides a shield against the cytotoxic effects of anatase.
Figure 2.. Relative viability (%) (y-axis) in the presence of 2.0 mg.mL-1 anatase compared to anatase-free controls as a function of time (min) (x-axis) for washed wild type of Gram-positive NCIB3610 and Gram-negative PAO1 cells (left), and for the corresponding mutants, yhxBΔ and Δ-psl respectively (right). Note reduced cell viability of Gram-negative cells compared to Gram-positive cells, indicating that the former have a cell-wall structure that is more robust towards the cytotoxic effect of anatase.
Publications
- Xu J., H., W., J. & Sahai, N. (2011). Evolution of bacterial biofilms as armor against mineral toxicity. Astrobiology.
- Xu, J., Sahai, N. & A, S.M.A. (In Preparation). Formation mechanisms of reactive oxygen species in aqueous suspensions of oxide minerals: Implications for organic and cellular evolution on early Earth and extra-terrestrial worlds. Astrobiology.
- Zhu, C., Xu, J., Hickey, W.J. & Sahai, N. (2011, In Preperation). Role of cell wall structure and extra-cellular polymeric substances (EPS) in shielding against specific mineral toxicity: Implications for cell surface evolution. Astrobiology.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Jie Xu
Doctoral Student
Chunxiao Zhu
Graduate Student
-
RELATED OBJECTIVES:
Objective 3.4
Origins of cellularity and protobiological systems
Objective 5.1
Environment-dependent, molecular evolution in microorganisms
Objective 7.1
Biosignatures to be sought in Solar System materials