2011 Annual Science Report
 University of Wisconsin
Reporting | SEP 2010 – AUG 2011
University of Wisconsin
Reporting | SEP 2010 – AUG 2011
Project 2A: Estimation of Pre-Biotic Amino Acids Delivery to Earth by Carbonaceous Chondrite Meteorites
Project Summary
The role of mineral surfaces in extraterrestrial organic synthesis, pre-biotic chemistry, and the early evolution of life remains an open question. Mineral surfaces could promote synthesis, preservation, or degradation of chiral excesses of organic small molecules, polymers, and cells. Different minerals, crystal faces of a mineral, or defects on a face may selectively interact with specific organics, providing an enormous range of chemical possibilities. We focus here on amino-acid isomer adsorption, conformation, and racemization on minerals representing primitive and altered peridotite found in chondritesand on planetary bodies. The study is inspired by the discovery of an excess of L-isovaline, a non-biologic amino acid, in a few carbonaceous meteorites by (Glavin and Dworkin, 2009), which suggests that the chiral nature of biology may have been due to excess L-amino acids delivered pre-biotically by meteorites.
In the present year of funding, we expanded our study to determine the conformation and binding modes of the acidic amino-acids, glutamate (Glu) and aspartate (Asp), adsorbed on model oxide, γ-Al2O3, using Attenuated Total Reflectance-Fourier Transform Infra-Red Spectroscocpy (ATR-FTIR) and the Triple Layer surface complexation model fits to bulk adsorption data over a wide range of pHs and amino-acid concentrations.
We also examined adsorption of L- and D- Glu, Asp, and a non-biological; amino acid, iso-valine on peridotite and serpentinte as pristine and altered analogs of carbonaceous chondrites, using LC-FD/ToF-MS. Preliminary results do not indicate preferential adsorption of either L- or D-amino-acids on peridotite and serpentinite, within detection limits. More detailed studies are required to improve the sensitivity and accuracy of the experiments involving the chiral amino-acids adsorption on chondritic analog materials.
The project addresses NASA Astrobiology Institute’s (NAI) Roadmap Goal 3 of understanding how life emerges from cosmic and planetary precursors , and Goal 4 of understanding organic and biosignature preservation mechanisms. The work is relevant to NASA’s Strategic Goal of advancing scientific knowledge on the origin and evolution life on Earth and potentially elsewhere, and of planning future Missions by helping to identify promising targets for the discovery of organics.
Project Progress
Between 4.8 and 3.8 Ga, the earth experienced periods of bombardments of meterioritic material including as carbonaceous chondrites. These meteorites contained organic compounds such as amino acids (AA), and are composed of 2 – 3% Al2O3 (Jarosewich, 1990). The Al2O3surface contains >AlO- binding sites for adsorption of AAS. The γ-Al2O3 phase has a high surface area, 26.6 m2/g and is a good starting point to establish techniques for studying amino-acid adsorption onto meteorites.
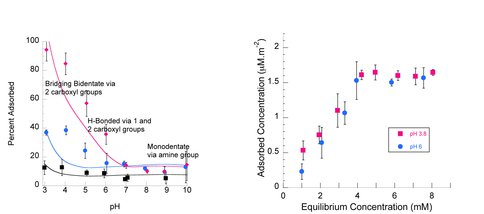
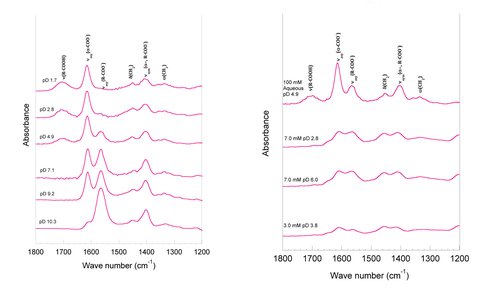
We have previously determined, using bulk adsorption data (pH-edges and adsorption isotherms), that the maximum adsorption density of both Glu and Asp on γ-Al2O3 is 1.6 μM / m2 (Figure 1). In the 2010-2011 year of funding, we used ATR-FTIR to determine how strongly the Glu and Asp are adsorbed on the γ-Al2O3 surface, by determining the binding coordination (modes of binding) (Figure 2). We used these binding modes to determine the stoichiometry of adsorption reactions (number of Glu or Asp molecules adsorbed per surface site). These stoichiometries then guided our Triple Layer Model Surface Complexation fits to the bulk adsorption data, resulting in adsorption equilibrium constants for each amino-acid in each biding mode (Figure 1). Both Glu and Asp were found to adsorb in the following geometries depending on pH: bridging bidentate via both carboxylate groups at acidic pH, H-bonded via one or two carboxylate groups at mid-pH range, and monodentate, electrostatic binding via the amine group above the PZC of alumina (pH > 9).
Having established the ability to determine adsorption quantities and binding modes of the amino-acids on γ-Al2O3, we extended our study to adsorption of L- and D-amino-acids on peridotite (Kilbourne Hole, Texas) and serpentinte (Troodos Massif, Cyprus), respectively, as pristine and altered analogs of carbonaceous chondrites. The results of adsorption of L- and D- Glu, and L- and-D- iso-valine (a non-biological amino-acid) on crushed using LC-FD/ToF-MS. Preliminary results seemed to show an enrichment of L-Glu in the solution when there was an excess of D-Glu in the starting solution. Both rocks resulted in -10% L-enrichment to + 10% L-enrichment of the solution. Thus, the rocks seemed to preferentially adsorb the D-isomer of Glu when the D- form was present in excess in the solution, or a net enrichment of 20%. However, the results were ambiguous for L- and D- iso-valine on both rocks. A net enrichment of L-iso-valine of only ~ 2.6% was seen in solution, when the starting solution was enriched in D-iso-valine. It is unclear at present, whether these results are accurate and bear repeating to confirm. Improved method development is, likely, required.
Graduate student, Edward Greiner, completed his M.S. thesis, based on this project, in August 2011 at University of Wisconsin-Madison. The results for the γ-Al2O3 study are currently being written up for publication.
Figure 1.. pH edges at L-Glu concentrations of 1.0, 0.1, and 0.01 mM (left), and adsorption isotherms for L-Asp at pH 3.8 and pH 6.0 on γ-Al2O3. The pH edges are fit to the Triple Layer Surface Complexation Model using FitEql 4.0 (solid curves) using binding geometries (i.e., adsorption reaction stoichiometires) informed by ATR-FTIR spectra for L-Glu adsorbed on γ-Al2O3 over a wide range of pHs.
Figure 2.. Infrared spectra of L-Glu (100 mM) in solution over a range of pD values (left), and of L-Glu adsorbed to a thin film of g-Al2O3 over a range of pD and total [L-Glu] (right). Frequency assignments of vibrational modes are indicated on the spectra.
Publications
- Greiner, E., Kumar, K., Giuffre, A., Sumit, M., Pedersen, J., Cleaves, J., Dworkin, J. & Sahai, N. (In Preparation). Adsorption of L-Glutamate and L-Aspartate from solution onto γ-Al2O3 surfaces. Langmuir.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Edward Greiner
Graduate Student
-
RELATED OBJECTIVES:
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules
Objective 4.1
Earth's early biosphere.