2011 Annual Science Report
 University of Wisconsin
Reporting | SEP 2010 – AUG 2011
University of Wisconsin
Reporting | SEP 2010 – AUG 2011
Project 1B: U-Th-Pb Geochronology and Fe Isotopes of the 3.4 Ga Marble Bar Chert Indicates Early Anoxygenic Photosynthesis
Project Summary
The origin of the spectacular red jasper (hematite+chert) at Marble Bar, Australia, has been a subject of debate for decades. Previous work has argued that oxidation occurred at the time of deposition 3.5 b.y. ago, and that the mechanism of oxidation was free oxygen in the atmosphere. This in turn would indicate that oxygenic photosynthesis had evolved by 3.4 Ga. By measuring Fe isotopes in the jasper, we can show that oxidation was extremely limited, and U-Th-Pb isotope geochronology on the same samples shows that the jasper precipitated form U-poor ocean water. This in turn indicates that there was no free oxygen in seawater at the time of deposition of the jasper. This in turn suggests that the mechanism was more likely to have been an anaerobic process, such as anoxygenic photosynthetic Fe(II) oxidation.
Project Progress
Evolution of Earth’s early atmosphere is critical to understanding the geologic history of the Earth, as well as the origin and evolution of life (e.g., Canfield, 2005). The occurrence of ferric oxides (hematite) in 3.4 Ga rocks from the Marble Bar area, Western Australia, has played an important role in interpreting the amount of free oxygen in the atmosphere of the ancient Earth. One of the key issues of using iron oxide minerals in Archean rocks to infer high O2 level in early Earth’s atmosphere is the age of the oxidation, and, if demonstrably ancient, the mechanism of oxidation. The Archean Biosphere Drilling Project (ABDP) was initiated to collect samples below weathering profiles in the hope of avoiding post-Archean weathering that may have affected near-surface samples. The first drill core (ABDP-1) was located at Marble Bar, and recovered both the hematite-bearing Marble Bar Chert (MBC) (Figure 1), as well as the Apex Basalt, from depths greater than 100 m. In Project 1C, we show that U-Th-Pb isotope geochronology of the Apex Basalt indicates oxidation of that unit by recent weathering, and not an ancient oxidation event in the Archean. Hoashi et al. (2009) studied the Marble Bar Chert, and argued that hematite is a primary mineral formed in the Archean, which they in turn interpreted to reflect high atmospheric O2 in the early Archean.
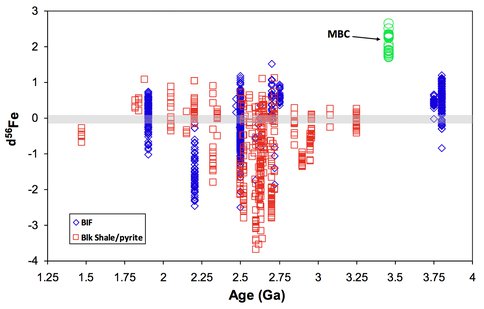
We revisited the same ABDP-1 drill core studied by Hoashi et al. (2009), using U-Th-Pb geochronology and Fe isotope geochemistry. Our results indicate that the Marble Bar Chert was deposited from a Fe-rich, but U-poor ocean. The δ56Fe values of hematite in MBC range between 1.71‰ and 2.63‰, which are the most positive δ56Fe dataset that has ever been reported from natural rocks (Figure 2). Hydrothermal sources of aqueous Fe2+ should have had a δ56Fe value of around 0‰ (Johnson et al., 2008), and therefore the very high δ56Fe values of hematite in MBC do not support the hypothesis proposed by Hoashi et al. (2009) that the ocean water at 3.4 Ga was oxygenated. If Archean ocean water was oxygenated, Fe2+ from hydrothermal vents would have been quantitatively oxidized, producing little to zero Fe isotope fractionation between Fe-oxide and aqueous Fe2+. In contrast, the high δ56Fe values of hematite in MBC requires partial oxidation of aqueous Fe2+, and, given the ~3‰ fractionation between oxides and aqueous Fe2+ (Johnson et al., 2002), less than 10% Fe oxidation occurred. This suggests that the oxidant was extremely limited.
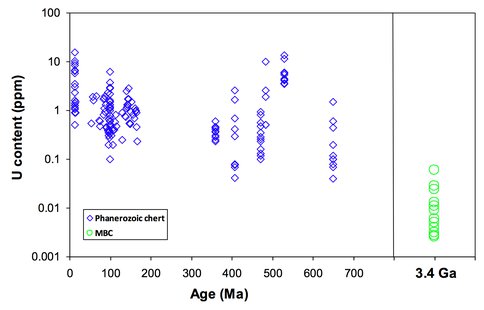
In addition, U concentrations of the MBC samples are over one order of magnitude lower than those of Phanerozoic cherts (Figure 3), indicating lower U content of Archean ocean water than modern ocean water. This also indicates low O2-level in Archean atmosphere because U is mobilized in oxidized form. We therefore conclude that the mechanism of oxidation was not interaction with free oxygen, but may have involved anoxygenic photosynthetic Fe(II) oxidation.
Figure 1. Figure 1. Outcrop of the 3.4 Ga Marble Bar Chert, Pilbara Craton, Australia. The hematite-rich chert (jasper) that was studied is the red layers.
Figure 2. Figure 2. Temporal variation in δ56Fe values for minerals and rocks from the Archean and Proterozoic sedimentary rocks. Gray bar denotes the isotopic baseline for bulk earth.
Figure 3. Figure 3. Comparison of U concentration in Phanerozoic cherts and MBC.
Publications
- Li, W., Beard, B.L., Van Kranendonk, M. & Johnson, C. (2012). U-Th-Pb isotope data and stable Fe isotopes indicate formation of the 3.4 Ga Marble Bar Chert from anoxic seawater. Nature.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Brian Beard
Co-Investigator
Martin Van Kranendonk
Collaborator
Weiqiang Li
Postdoc
-
RELATED OBJECTIVES:
Objective 4.1
Earth's early biosphere.
Objective 6.1
Effects of environmental changes on microbial ecosystems
Objective 7.1
Biosignatures to be sought in Solar System materials