2011 Annual Science Report
 University of Hawaii, Manoa
Reporting | SEP 2010 – AUG 2011
University of Hawaii, Manoa
Reporting | SEP 2010 – AUG 2011
Ice Chemistry of the Solar System
Project Summary
The overall goals of this project are to understand the chemical evolution of the Solar System, in particular leading to the development of astrobiologically important molecules. This is being achieved by investigation the formation of key organic carbon-, hydrogen-, oxygen-, and nitrogen-bearing (CHON) molecules in ices of Kuiper belt objects by reproducing the space environment experimentally in a unique ultra-high vacuum surface scattering machine. During this reporting period, our team worked on six projects towards our research goal to better understand the ice-based astrochemistry of chemical synthesis for carbon-containing compounds within the solar system. The Keck Astrochemistry Laboratory was also completed during this reporting period.
Project Progress
“Laboratory Simulation of KBO Volaitle ices Under Ionizing Radiation: CO-N2 Ices”
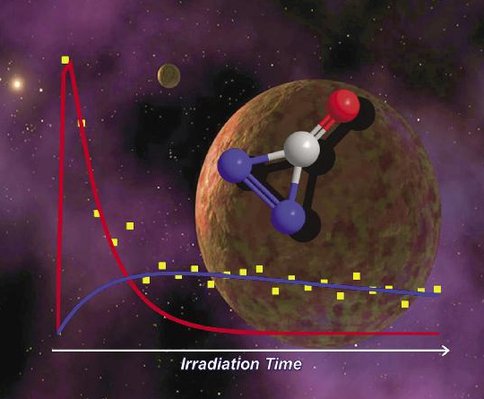
The exposure of icy Kuiper Belt Objects (KBOs) by ionizing radiation was simulated in this case of exposing carbon monoxide–nitrogen (CO-N2) ices by energetic electrons. The radiation-induced chemical processing was monitored on-line and in-situ via FTIR spectroscopy and quadupole mass spectrometry. Besides the array of carbon oxides being reproduced as in neat irradiated carbon monoxide (CO) ices studied previously, the radiation exposure at 10 K resulted in the formation of nitrogen-bearing species of isocyanato radical (OCN), linear (l-NCN), nitric oxide (NO), nitrogen dioxide (NO2), plus diazirinone (N2CO). The infrared assignments of these species were further confirmed by isotopic shifts. The temporal evolution of individual species was found to fit in first-order reaction schemes, prepping up the underlying non-equilibrium chemistry on the formation of OCN, l-NCN, and NO radicals in particular. Also unique to the binary KBO model ices and viable for the future remote detection is diazirinone (N2CO) at 1860 cm-1 (2ν5) formed at lower radiation exposure.
“Formation of Nitric Oxide and Nitrous Oxide in Electron-irradiated H218O/N2 Ice Mixtures—Evidence for the Existence of Free Oxygen Atoms in Interstellar and Solar System Analog Ices”
We investigated the irradiation of low temperature H218O/N2 ice mixtures with energetic electrons in an ultrahigh vacuum chamber. The newly formed species, such as nitric oxide (N18O), nitrous oxide (NN18O), hydrogen peroxide (H218O2) and hydrazine (N2H4), were identified in the experiments with infrared absorption spectroscopy and mass spectrometry. The results suggest that the unimolecular decomposition of water molecules within water ices at 10 K can lead to the formation of transient, suprathermal oxygen atoms. These oxygen atoms may play an important role in the formation of oxygen-containing biomolecules such as amino acids and sugar, as well as the decomposition of the biomolecules in the ices.
“Formation Formic Acid in Interstellar and Cometary Ices”
The present laboratory study simulated the abiotic formation of carboxylic acids (RCOOH) in interstellar and solar system model ices of carbon dioxide (CO2)–hydrocarbon mix CnH2n+2 (n=1–6). The pristine model ices were irradiated at 10 K under contamination-free, ultrahigh vacuum conditions with energetic electrons generated in the track of galactic cosmic-ray particles. The chemical processing of the ices was monitored by a Fourier transform infrared spectrometer and a quadrupole mass spectrometer during the irradiation phase and subsequent warm-up phases on line and in situ in order to extract qualitative (carriers) and quantitative (rate constants and yields) information on the newly synthesized species. Carboxylic acids were identified to be the main carrier, together with carbon monoxide (CO) and a trace of formyl (HCO) and hydroxycarbonyl (HOCO) radicals at 10 K. The upper limit of acid column density at 10K was estimated as much as (1.2±0.1)×1017 molecules cm−2 at doses of 17±2 eV molecule−1, or the yield of 39%±4% from the initial column density of carbon dioxide. The temporal column density profiles of the products were then numerically fit using two independent kinetic schemes of reaction mechanisms. Finally, we transfer this laboratory simulation to star-forming regions of the interstellar medium, wherein cosmic-ray-induced processing of icy grains at temperatures as low as 10 K could contribute to the current level of chemical complexity as evidenced in astronomical observations and in extracts of carbonaceous meteorites.
“Formation of D2-Water and D2-Carbonic Acid in Oxygen-Rich Solar System Ices via D+2 Implantation”
Molecular oxygen (O2) and carbon dioxide (CO2) ices were irradiated with energetic D+2 ions to simulate the exposure of oxygen-bearing solar system ices to magnetospheric H+2 and H+ ions and energetic protons from the solar wind. The experiments provided evidence on the incorporation of the implanted deuterium and inherent formation of D2-water (D2O) as well as D2-carbonic acid (D2CO3). In the molecular oxygen ices, the temporal profiles inferred that D2-water formation followed successive deuterium atom addition to atomic oxygen via a D-hydroxyl radical intermediate in the matrix. In the carbon dioxide ices, D2-carbonic acid was likely formed via successive deuterium atom reaction with cyclic carbon trioxide. These chemical processes have specific relevance to water formation on outer solar system bodies, such as the icy moons of Jupiter and Saturn, as well as possible implications for the formation of water on the lunar surface.
“Formation of Ozone in Oxygen-Rich Solar System Ices via Ionizing Radiation”
The irradiation of pure molecular oxygen (O2) and carbon dioxide (CO2) ices with 5 keV H+ and He+ ions was investigated experimentally to simulate the chemical processing of oxygen rich planetary and interstellar surfaces by exposure to galactic cosmic ray (GCR), solar wind, and magnetospheric particles. Deposited at 12 K under ultra-high vacuum conditions (UHV), the irradiated condensates were monitored on-line and in situ in the solid-state by Fourier transform infrared spectroscopy (FTIR), revealing the formation of ozone (O3) in irradiated oxygen ice; and ozone, carbon monoxide (CO), and cyclic carbon trioxide (c-CO3) in irradiated carbon dioxide. In addition to these irradiation products, evolution of gas-phase molecular hydrogen (H2), atomic helium (He) and molecular oxygen (O2) were identified in the subliming oxygen and carbon dioxide condensates by quadrupole mass spectrometry (QMS). Temporal abundances of the oxygen and carbon dioxide precursors and the observed molecular products were compiled over the irradiation period to develop reaction schemes unfolding in the ices. These reactions were observed to be dependent on the generation of atomic oxygen (O) by the homolytic dissociation of molecular oxygen induced by electronic, Se, and nuclear, Sn, interaction with the impinging ions. In addition, the destruction of the ozone and carbon trioxide products back to the molecular oxygen and carbon dioxide precursors was promoted over an extended period of ion bombardment. Finally, destruction and formation yields were calculated and compared between irradiation sources (including 5keV electrons) which showed a surprising correlation between the molecular yields (~103–104 molecules eV-1) created by H+ and He+ impacts. However, energy transfer by isoenergetic, fast electrons typically generated ten times more product molecules per electron volt (~102–103 molecules eV-1) than exposure to the ions. Implications of these findings to Solar System chemistry are also discussed.
“Forming D2-Water & D2-Carbonic Acid in Oxygen-Rich Solar System Ices via D2+Irradiation”
Molecular oxygen (O2) and carbon dioxide (CO2) ices were irradiated with energetic D2+ ions to simulate the exposure of oxygen-bearing solar system ices to magnetospheric H2+ and H+ ions and energetic protons from the solar wind. The experiments provided evidence on the incorporation of the implanted deuterium and inherent formation of D2-water (D2O) as well as D2-carbonic acid (D2CO3). In the molecular oxygen ices, the temporal profiles inferred that D2-water formation followed successive deuterium atom addition to atomic oxygen via a D-hydroxyl radical intermediate in the matrix. In the carbon dioxide ices, D2-carbonic acid was likely formed via successive deuterium atom reaction with cyclic carbon trioxide. These chemical processes have specific relevance to water formation on outer solar system bodies, such as the icy moons of Jupiter and Saturn, as well as possible implications for the formation of water on the lunar surface.
Cover article for Physical Chemistry, Chemical Physics (Kaiser et al. Laboratory simulation of Kuiper belt object volatile ices under ionizing radiation: CO-N2 Ices as a Case Study. PCCP Volume 13(35), September 2011: pp. 15729-16108.
Overview of the W. M. Keck Research Laboratory in Astrochemistry located in the chemistry building on the Univ. of Hawaii at Manoa campus.
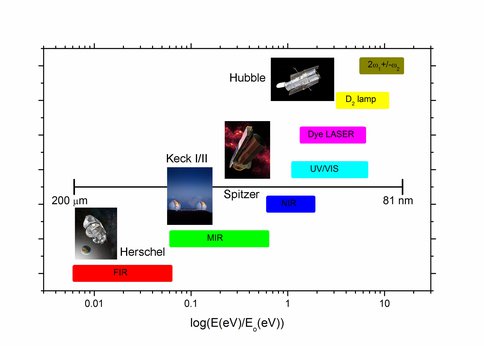
Analytical light sources from the infrared (IR) to the vacuum ultraviolet (VUV) in the simulation chamber compared to currently operating space probes.
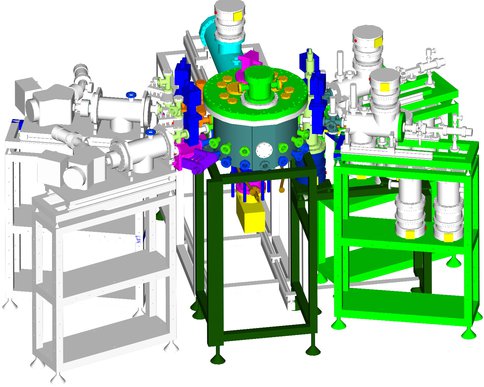
Assembly drawing of the Keck Astrochemistry Laboratory facility.
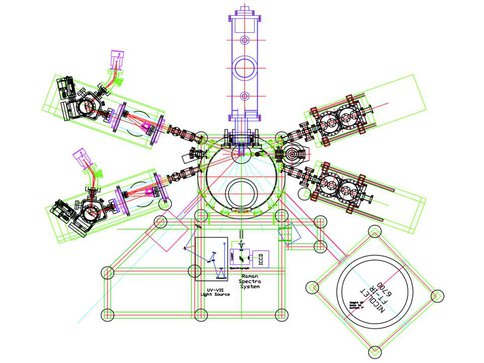
Top view of the new surface scattering machine.
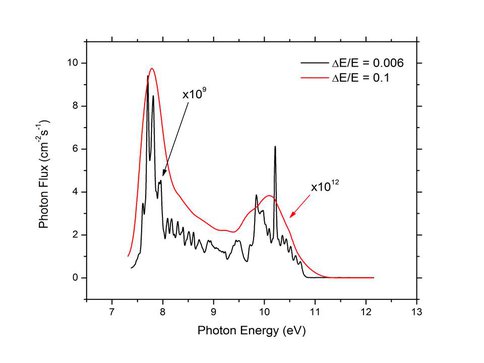
Tunable vacuum UV source high and low resolution spectra.
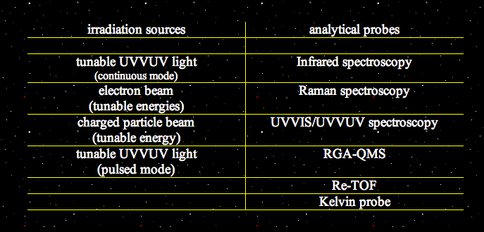
Summary of facility capabilities
Publications
-
Ennis, C. P., Bennett, C. J., & Kaiser, R. I. (2011). On the formation of ozone in oxygen-rich solar system ices via ionizing radiation. Physical Chemistry Chemical Physics, 13(20), 9469. doi:10.1039/c1cp20434c
-
Ennis, C., Bennett, C. J., Jones, B. M., & Kaiser, R. I. (2011). FORMATION OF D 2 -WATER AND D 2 -CARBONIC ACID IN OXYGEN-RICH SOLAR SYSTEM ICES VIA D + 2 IRRADIATION. The Astrophysical Journal, 733(2), 79. doi:10.1088/0004-637x/733/2/79
-
Kim, Y. S., & Kaiser, R. I. (2011). ON THE FORMATION OF AMINES (RNH 2 ) AND THE CYANIDE ANION (CN – ) IN ELECTRON-IRRADIATED AMMONIA-HYDROCARBON INTERSTELLAR MODEL ICES. The Astrophysical Journal, 729(1), 68. doi:10.1088/0004-637x/729/1/68
-
Kim, Y. S., Zhang, F., & Kaiser, R. I. (2011). Laboratory simulation of Kuiper belt object volatile ices under ionizing radiation: CO–N2 ices as a case study. Physical Chemistry Chemical Physics, 13(35), 15766. doi:10.1039/c1cp20658c
-
Zheng, W., Kim, Y. S., & Kaiser, R. I. (2011). Formation of nitric oxide and nitrous oxide in electron-irradiated H218O/N2 ice mixtures—evidence for the existence of free oxygen atoms in interstellar and solar system analog ices. Physical Chemistry Chemical Physics, 13(35), 15749. doi:10.1039/c1cp20528e
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Chris Bennett
Project Investigator
Brant Jones
Co-Investigator
Ralf Kaiser
Co-Investigator
-
RELATED OBJECTIVES:
Objective 1.1
Formation and evolution of habitable planets.
Objective 2.2
Outer Solar System exploration
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules
Objective 3.3
Origins of energy transduction
Objective 6.2
Adaptation and evolution of life beyond Earth
Objective 7.1
Biosignatures to be sought in Solar System materials