2011 Annual Science Report
 Rensselaer Polytechnic Institute
Reporting | SEP 2010 – AUG 2011
Rensselaer Polytechnic Institute
Reporting | SEP 2010 – AUG 2011
Project 7: Prebiotic Chemical Catalysis on Early Earth and Mars
Project Summary
The “RNA World” hypothesis is the current paradigm for the origins of terrestrial life. Our research is aimed at testing a key component of this paradigm: the efficiency with which RNA molecules form and grow under realistic conditions. We are studying abiotic production and polymerization of RNA by catalysis on montmorillonite clays. The catalytic efficiency of different montmorillonites are determined and compared, with the goal of determining which properties distinguish good catalysts from poor catalysts. We are also investigating the origin of montmorillonites, to test their probable availability on the early Earth and Mars, and the nature of catalytic activity that could have led to chiral selectivity on Earth.
Project Progress
(a) Ongoing investigations of catalytic clays
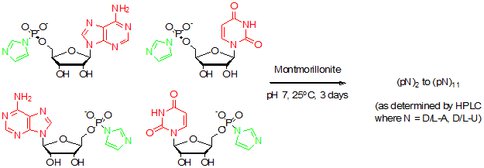
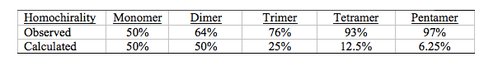
Previous studies from our laboratory have demonstrated that selected montmorillonite clay minerals catalyze the formation of RNA oligomers from activated mononucleotides (1). However, demonstration of the origin of molecular homochirality in RNA under prebiotic simulated conditions remains elusive. It is believed that individual mononucleotides, formed by prebiotic processes on the primitive Earth, were likely present in racemic mixtures but only D-ribose is present in naturally occurring RNA. The question is how was chiral selection introduced into the prebiological system? We investigated the quaternary reactions of D, L-ImpA with D, and L-ImpU on montmorillonite (Figure 1). The chain length of these oligomers increased from 9-mer to 11-mer as observed by HPLC, with a concomitant increase in the yield of linear dimers and higher oligomers. 12 linear dimers and 3 cyclic dimers were isolated and characterized from the quaternary reaction. The homochirality and regioselectivity of dimers were 64% and 72%, respectively. Of the 16 trimers detected, 10 were homochiral with an overall homochirality of 76% (2).
Fig.1. Reaction of the racemic 5’-phosphorimidazolides of adenosine and uridine on Na+-montmorillonite.
Encouraged by favorable homochiral excesses of dimer and trimer, the study was extended to the analysis of higher oligomers. The tetramer and pentamer of the quaternary reaction were separated into 26 and 22 isomers, respectively, on a reverse phase column. Their co-elution with those formed in the binary reaction (i.e. D-ImpA and D-ImpU on Na+-montmorillonite) revealed 93% and 97% homochirality of the tetramer and pentamer, respectively (see Table 1 below). These results suggest that Na+-montmorillonite could not only catalyze the prebiotic synthesis of RNA but it also facilitate homochiral selection (3). Further investigation of the MALDI MS of RNA oligonucleotides found enhanced signals as well as for other anionic biopolymers including DNA oligonucleotides and heparin. Among more general analytical applications, the results are particularly relevant to rapid screening of abiotic RNA polymerization toward elucidation of pathways to life on Earth (4).
In an ongoing study on the investigation of catalytic activity of Bentonites collected wordwide, we have identified several excellent/good catalysts located mainly in the northern United States. Follow-up investigations have led to southern Manitoba where two excellent catalysts and a good catalyst have been recognized from the Odanah member of the Pierre Shale (5). Additional testing will be done in an attempt to recognize if the Odanah member Bentonite consistently provides catalytic properties and to determine if these properties may be attributed in part to the underlying Paleozoic basement rock.
Nearly 40 montmorillonite-rich Bentonites, whose isotopic ages and geologic settings were known, have been chemically analyzed (X-ray fluorescence; XRF) for 30 major-, minor, and trace elements. These chemical analyses have been studied in the context of their level of catalytic activity and geologic settings of formation. X-ray diffraction (XRD) analyses of these montmorillonite-rich Bentonites are continuing. Based on this year’s work, it appears that a narrow range of both chemical compositions and geologic settings are associated with clays observed to be excellent catalysts for RNA oligomerization. In addition, the composition of volcanic ashes that were chemically altered to form these catalytically active Bentonites has been constrained. Those magmatic compositions have been compared with constraints on the compositions of Hadean magmas inferred from analysis of Hadean zircons from the Jack Hills. A paper reporting these results is in preparation.
Although, montmorillonite catalyzed RNA synthesis is familiar, its mechanism is still not well understood. To provide a clearer picture of the mechanism, we have observed that the extent of catalysis depends not only upon the magnitude of the negative charge on the montmorillonite lattice and the number of cations associated with it, but also on the pH at which the reaction is promoted and the nature of the cation (6). Isotherm and catalysis studies have been extended to provide binding information and catalytic outcomes over a wide pH range. The optimal binding occurs in the region of maximal oligomer formation.
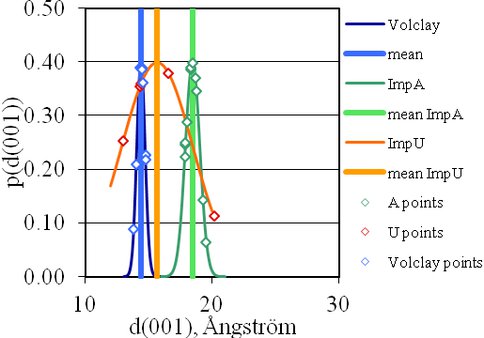
X-ray diffraction studies revealed changes in layer separations of montmorillonite as reaction occurs (Figure 2). The application of the Scherrer equation to the X-ray diffraction data showed differences in domain size. Modeling of the size of the activated nucleotide monomers and the charge on the montmorillonite surface provided an interpretation of how these factors influence adsorption. We have observed that the extent of catalysis depends not only upon the magnitude of the negative charge on the montmorillonite lattice and the number of cations associated with it, but also on the pH at which the reaction is promoted.
Fig.2. d(001) spacing for Volclay with and without admixed activated nucleotide.
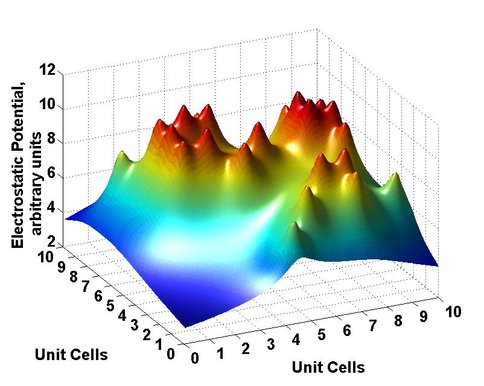
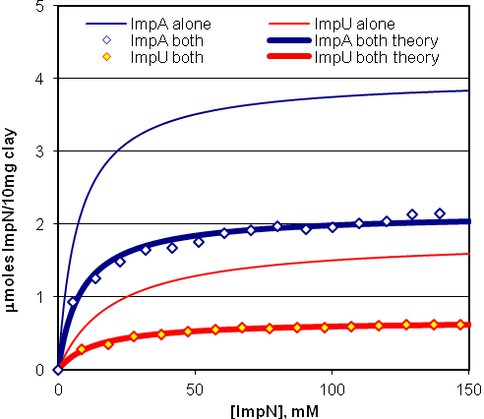
In turn, regions of maximum catalytic activity can be envisaged associated with the regions of lesser charge on the clay surfaces (Figure 3). The isotherm (Figure 4) and catalysis studies have been extended to provide binding information and catalytic outcomes over a wide pH range. The optimal binding occurs in the region of maximal oligomer formation. X-ray diffraction studies revealed changes in layer separations of montmorillonite as reaction occurs. This research provides a basis for further understanding of the physical processes in the mechanism of this catalysis.
Fig.3. The simple potential surface from22 random isomorphous substitutions in 100 unit cells. Adsorption and reaction may occur in the potential wells, but not in the regions of higher potential. D-ImpU will be lower in the wells than D-ImpA.
Fig.4. Langmuir isotherms for D-ImpA and D-ImpU on Volclay, both separately and together.
(b) Terahertz spectroscopy applied to studies of catalytic clays
In a new development, the catalytic chemistry group is collaborating with Ingrid Wilke and Saroj Nayak (Department of Physics and members of the Center for Terahertz Research at RPI). The goal is to investigate the potential of terahertz (THz) spectroscopy for the detection of prebiotic chemistry in clay minerals and for the non-destructive subsurface characterization of biomarkers in surface rocks and soils. The idea stems from the observation that clay minerals are rather transparent in this frequency range whereas biomolecules exhibit characteristic absorptions of radiation at these frequencies. The experimental design is guided by recent advancements in THz spectroscopy instrumentation and improved understanding of the molecular dynamics of biomolecules in the THz region. If THz spectroscopy can be successfully employed to study the adsorption of biomolecules in clays, it will benefit multiple NASA Astrobiology objectives, including robotic planetary missions to Mars and elsewhere. A proposal based on this work was submitted to the NAI Director’s Discretionary Fund in 2011 (decision pending).
References
1. Prakash C. Joshi, Michael F. Aldersley, John W. Delano and James P. Ferris (2009) Mechanism of montmorillonite catalysis in the Formation of RNA oligomers. Journal of the American Chemical Society 131, 13369 – 13374.
2. Prakash C. Joshi, Michael F. Aldersley and James P. Ferris (2011) Homochiral selectivity in RNA synthesis on montmorillonite-catalyzed reactions of D, L-purine and pyrimidine nucleotides, Origins of Life and Evolution of Biosphere, 41, 213-236.
3. Prakash C. Joshi, Michael F. Aldersley and James P. Ferris (2011) Progress in demonstrating total homochiral selection in montmorillonite-catalyzed RNA synthesis, Biochemical and Biophysical Research Communications, 413, 594-598.
4. Lauren M. Cassidy, Yingying Dong, Prakash C. Joshi, Michael F. Aldersley, James P. Ferris and Linda B. McGown (2011) Signal enhancement of abiotically-synthesized RNA oligonucleotides and other biopolymers using unmodified fused silica in MALDI-MS, Journal of the American Society for Mass Spectrometry, 22, 1100-1104.
5. M. F. Aldersley, J. D. Bamburak, P. C. Joshi, J. Thompson, J. W. Delano, and J. P. Ferris (2011) GS-X12 Evaluation of Manitoba Bentonites in the catalysis of RNA synthesis by montmorillonite, Manitoba Innovation, Energy and Mines 2011, Manitoba Geological Survey, Report of Activities (In Press)
6. Michael F. Aldersley, Prakash C. Joshi, John W. Delano, Jonathan Price and James P. Ferris (2011) Mechanism of montmorillonite catalysis in the Formation of RNA oligomers. Part II. Applied Clay Science, 54, 1-14.
Publications
-
Cassidy, L. M., Dong, Y., Joshi, P. C., Aldersley, M. F., Ferris, J. P., & McGown, L. B. (2011). Signal Enhancement of Abiotically-Synthesized RNA Oligonucleotides and other Biopolymers using Unmodified Fused Silica in MALDI-MS. Journal of The American Society for Mass Spectrometry, 22(6), 1100–1104. doi:10.1007/s13361-011-0113-0
-
Joshi, P. C., Aldersley, M. F., & Ferris, J. P. (2010). Homochiral Selectivity in RNA Synthesis: Montmorillonite-catalyzed Quaternary Reactions of D, L-Purine with D, L- Pyrimidine Nucleotides. Orig Life Evol Biosph, 41(3), 213–236. doi:10.1007/s11084-010-9222-1
-
Joshi, P. C., Aldersley, M. F., & Ferris, J. P. (2011). Progress in demonstrating total homochiral selection in montmorillonite-catalyzed RNA synthesis. Biochemical and Biophysical Research Communications, 413(4), 594–598. doi:10.1016/j.bbrc.2011.09.008
-
Joshi, P. C., Aldersley, M. F., Delano, J. W., & Ferris, J. P. (2009). Mechanism of Montmorillonite Catalysis in the Formation of RNA Oligomers. Journal of the American Chemical Society, 131(37), 13369–13374. doi:10.1021/ja9036516
- Aldersley, M.F. (2011). Catalysis of an exclusive RNA Dimer Synthesis by Montmorillonite. Origins 2011 conference. Montpellier, France.
- Aldersley, M.F. (2011). The Role of Montmorillonite in its Catalysis of RNA Synthesis. Origins 2011 conference. Montpellier, France.
- Aldersley, M.F., Bamburak, J.D., Joshi, P.C., Thompson, J., Delano, J.W. & Ferris, J.P. (2011, In Press). GS-X12 Evaluation of Manitoba Bentonites in the catalysis of RNA synthesis by montmorillonite, Manitoba Innovation, Energy and Mines 2011, Manitoba Geological Survey, Report of Activities.
- Ferris, J.P. (2011). Progress in Studies on the RNA World. Origins 2011 conference. Montpellier, France.
- Josh, P.C. (2011). Progress Towards Total Homochirality in Prebiotic Synthesis. Origins 2011 conference. Montpellier, France.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
John Delano
Co-Investigator
Linda McGown
Co-Investigator
Ingrid Wilke
Co-Investigator
Saroj Nayak
Collaborator
Michael Aldersley
Research Staff
Lauren Cassidy
Graduate Student
John Grossman
Undergraduate Student
Alex Meola
Undergraduate Student
Matthew Moellman
Undergraduate Student
-
RELATED OBJECTIVES:
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules