2011 Annual Science Report
 Rensselaer Polytechnic Institute
Reporting | SEP 2010 – AUG 2011
Rensselaer Polytechnic Institute
Reporting | SEP 2010 – AUG 2011
Project 6: The Environment of the Early Earth
Project Summary
This project involves the development of capabilities that will allow scientists to obtain information about the conditions on early Earth (3.0 to 4.5 billion years ago) by performing chemical analyzes of crystals (minerals) that have survived since that time. When they grow, minerals incorporate trace concentrations of ions and gaseous molecules from the local environment. We are conducting experiments to calibrate the uptake of these “impurities” that we expect to serve as indicators of temperature, moisture, oxidation state and atmosphere composition. To date, our focus has been mainly on zircon (ZrSiO4), but we have recently turned our attention to quartz as well.
Project Progress
In the last year, significant progress has been made towards the completion of several of our main research themes. These projects were originally outlined in section 7.2 of our 2008 NAI proposal (uptake of 'indicator species’ in ancient zircons and other silicates). The following discussion presents significant contributions or milestones achieved during the reporting period.
Ce and Eu anomalies in zircon (ZrSiO4): indicators of oxygen fugacity and melt composition
Results of our experimental calibration demonstrate that Ce concentrations in zircons (and Ce anomalies) correlate with higher oxygen fugacities and lower crystallization temperatures. The calibration was applied to natural zircons of known provenance and independently constrained magma oxygen fugacity (e.g., lunar zircons and terrestrial mantle-derived crystals). These comparisons demonstrate that the zircon Ce-oxygen fugacity sensor accurately predicts the oxygen fugacity of melts from which individual zircons crystallize. The calibration was then applied to zircons determined by the U-Pb method to be older than 4 billion years. Results indicate that oxygen fugacities preserved in ancient zircons are broadly similar to those observed from present-day terrestrial magmas. In addition >4.0 Ga zircons that have chemical characteristics indicative of a mantle melt source indicate an oxygen fugacity close to the fayalite–magnetite–quartz (FMQ) buffer, which is similar to the oxidation state of the Archean and present day mantle. Knowledge of the oxidation state of magmas also allows the speciation of volatile gases to be calculated. Since the volatile species outgassing from the Hadean mantle probably played an important role in determining the composition of the early atmosphere, estimating the oxidation state of the Hadean mantle would mean that a first-order constraint could be placed on the composition of the early atmosphere. The calculated species of gases emanating for the Hadean mantle were H2O, N2 SO2, and CO2. Results of this study will be published in Nature this fall.
In addition, characterization of Eu anomalies vs. oxygen fugacity has now been completed. Eu anomalies in zircon are often attributed to the crystallization of plagioclase feldspar (which preferentially sequesters Eu relative to other REEs because Eu can be divalent and substitute for Ca2+) prior to or during zircon crystallization. Eu anomalies become more negative at lower oxygen fugacities and results demonstrate that plagioclase is not a requisite for the presence of Eu anomalies in zircon. A manuscript that discusses the constraints that can be on melt composition (e.g., the presence of plagioclase) is in preparation.
Biosignature mimics – mass dependent carbon isotope fractionation
The abundances and isotopic compositions of C, S, N, H as well as redox-sensitive elements such as Fe, Mn or Mo are used to trace biological activity in ancient sedimentary rocks. For example, biological activity is known to modify (specifically, to make lighter) the carbon isotopic ratio (δ13C), so light carbon signatures in minerals of ancient rocks are commonly attributed to the metabolic activity of living organisms. That said, it has been shown that inorganic processes such as diffusive transport of atoms in Earth materials can also fractionate isotopes. Therefore, it imperative to determine whether abiological processes can fractionate isotopes. We undertook an experimental study of carbon diffusion in the grain boundaries of rocks and in Fe metal, in order determine the effect of isotopic mass on diffusion.
Our ion probe work on these experiments demonstrated that C isotopes are fractionated as they diffuse through an Fe polycrystalline rod. In particular, 12C diffuses faster through the rod than 13C. In a separate experiment, we also measured the 13C/12C ratio of small Fe particles dispersed along grain boundaries of oxide minerals. We found that the13C/12C ratio of the dispersed particles is enriched in 12C when compared to the source. One interpretation of this data is that 12C has a higher diffusivity than 13C in oxide grain boundaries. While further exploration is required, these results indicate that it may be possible to yield isotopically light carbon signatures in rocks via transport of C-isotopes.
Trace element incorporation into quartz
Quartz (SiO2) arguably represents the defining mineral of the continental crust, and although characterized by compositions close to purity, is known to contain a number of trace elements (e.g., Al, Ti, Fe, P, Li, K, H, etc). This is true of ancient crustal rocks (e.g., Jack Hills, the Acasta gneiss, Witwatersrand conglomerate) and modern crustal rocks (e.g., granite and hydrothermal quartz). Equally important and pertinent to this research program, quartz is known to show a number of systematic saturation trends (Ti-in-quartz, Wark and Watson, 2006) that make it useful as a thermobarometer.
The primary focus of this research has been to constrain Aluminum (Al)-solubility over a range of P-T-X conditions in order to establish if concentration can be used as an indicator of crystallization environment in ancient quartz-bearing rocks.The experimental study incorporates two basic approaches: (1) crystallizing quartz from systems of different composition and activity buffers ( at defined P-T conditions), and (2) infinite source Al-in-quartz diffusion experiments, measured by nuclear reaction analysis (NRA). The first technique provides the raw calibration required of thermobarometry and the second establishes how robust or easily reset the system is.
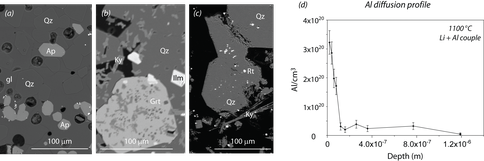
Figure 1: (a) representative BSE images of experimental run products from Al-in-quartz experiments incorporating Al2O3-SiO2-TiO2-H2O and Al2O3-CaO-P2O5-SiO2-TiO2 systems at a common temperature. (b) NRA analysis of infinite source diffusion experiment incorporating spodumene-quartz pair.
Results indicate that Al concentration, at crustal conditions (e.g., 10-25 kbar, 600-900 °C, fO2 = FMQ), show systematic trends suited to thermobarometry. Moreover, at vapor saturated conditions, results suggest that a range of compositions result in consistent Al-saturation (regardless of the activity of a number of potential charge balancing elements). Diffusion results demonstrate that Al diffusivity is exceedingly slow in quartz (comparable to Ti), meaning that diffusive resetting is limited except at the temperature extremes of crustal environments.
Eu2+/Eu3+ in Apatite [Ca5(PO4)3(OH)] and Whitlockite [Ca9(Mg,Fe2+)(PO4)6(PO3OH)]
Apatite and whitlockite are common accessory phases in a number of terrestrial bodies within the solar system, including the Earth, the Moon and Mars. Among the various trace elements that substitute into these phosphate minerals, the Rare Earth Elements (REE) are known to be both compatible and exist at readily detectable concentrations (Watson and Green, 1981). The REE concentration of accessory phases, particularly when chondrite normalized, is a particularly useful tool for petrologists as the similar chemical behavior of these elements (which generally show smooth trends from La to Lu which correspond to decreasing ionic radii) can be used to elucidate something of the melt source. There are two exceptions to the general smooth trend among the lanthanides – Europium (Eu) and Cerium (Ce), with these two elements displaying polyvalent behavior under specific conditions.
For the purpose of this research we studied the polyvalent behavior of Eu in phosphates crystallized at 1 atmosphere, and varying temperature-fO2 conditions. The choice of Eu was based on previous estimates of the Eu valence speciation curve (e.g., Papike et al., 2005; Wadhwa, 2008) and the fO2 of different terrestrial bodies.
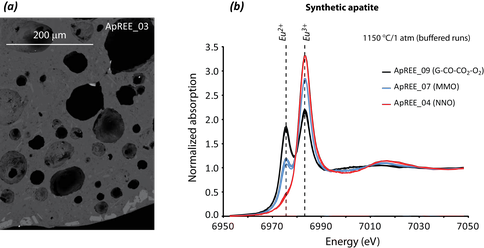
Experiments incorporated solid-state buffers (ranging from Fe3O4-Fe2O3 to C-CO2-CO-O2, or spanning ~12 log units) and phosphate-saturated basaltic melts similar to lunar KREEP. Experimental run products, including minerals and glass, were analyzed via XANES spectroscopy and clearly indicate that a distinct T-fO2 trend can be calibrated. In turn this calibration may be applied to any near-basaltic melt that is obtained from any terrestrial body within the solar system, making it a particularly useful and important experimental calibration.
Figure 2: (a) BSE image from phosphate-saturated basalt system, including (in decreasing brightness in grayscale) whitlockite, apatite, basaltic glass and plagioclase. (b) Representative Eu L3 XANES scans of apatite at 1150 °C/1 atm and different fO2 conditions (colors as indicated).
Isotopic fractionation by grain boundary diffusion
The isotopic ratio of sulfur in biogenic sulfide minerals is thought to be influenced by the metabolic chemistry of the “parent” organism. In general, metabolic reactions favor the lighter isotopes of biochemically important elements, so isotopically “light” S isotope ratios in sulfides are commonly taken as confirmation of a biogenic origin. However, certainty of a biogenic origin requires ruling out an abiotic mechanism to fractionate the isotopes of interest. A possible competing process of widespread occurrence in the host rocks of isotopically light minerals is grain boundary diffusion. The existence and magnitude of isotopic fractionation by grain boundary diffusion must be understood before a biotic origin may be firmly assigned to isotopically light minerals.
To measure the extent of isotopic fractionation by grain boundary diffusion, we use an experimental setup that consists of an isotopically enriched source which is juxtaposed with a prefabricated ‘matrix rock’ containing dispersed sink particles. Measurements of the isotopic ratios of sulfur in the sink particles will reveal any fractionation which occurred by grain boundary diffusion through the ‘matrix rock’. We have used a similar experimental design in studies of grain boundary diffusion of manganese and iron, which are additional important elements used to deduce the presence of biological activity. The experiments have established the feasibility of characterizing the process of grain boundary diffusion, but have not yet yielded data on isotope fractionation.
Publications
-
Trail, D., Thomas, J. B., & Watson, E. B. (2010). The incorporation of hydroxyl into zircon. American Mineralogist, 96(1), 60–67. doi:10.2138/am.2011.3506
-
Trail, D., Watson, E. B., & Tailby, N. D. (2011). The oxidation state of Hadean magmas and implications for early Earth’s atmosphere. Nature, 480(7375), 79–82. doi:10.1038/nature10655
- Mojzsis, S.J., Cates, N.L., Maier, A.C., Hopkins, M., Trail, D., Guitreau, M., Abramov, O. & Bleeker, W. (2011). The ca. 4.2-3.7 Ga History of the Acasta Gneiss Complex (Northwest Territories, Canada). Goldschmidt Conference.
- Tailby, N., Trail, D., Cates, N.L., Mojzsis, S.J., Bell, E.A., Harrison, T.M. & Watson, E.B. (2011). Direct measurement of Ce3+/Ce4+ and Eu2+/Eu3+ in Hadean zircons by XANES. Goldschmidt Conference.
- Tailby, N., Trail, D., Cates, N.L., Mojzsis, S.J., Bell, E.A., Harrison, T.M. & Watson, E.B. (2011, In Preparation). Direct measurement of Ce and Eu valence in Hadean zircons by XANES.
- Trail, D., Watson, E.B. & And Tailby, N.D. (In Preparation). Ce and Eu anomalies in zircon as proxies for the oxidation state of magmas.
- Trail, D., Watson, E.B. & Tailby, N. (2010). New experimental constraints for Hadean zircon source melts from Ce and Eu anomalies in zircon. American Geophysical Union.
- Trail, D., Watson, E.B. & Tailby, N. (2011). The oxidation state of Hadean melts and implications for the composition of Earth’s early atmosphere. Goldschmidt Conference.
- Watson, E.B., Mueller, T., Trail, D., Van Orman, J.A. & Papineau, D. (2011). C Diffusion in Fe: isotope effects and other complexities. American Geophysical Union.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Suzanne Baldwin
Co-Investigator
John Delano
Co-Investigator
Thomas Mueller
Collaborator
Nick Tailby
Postdoc
Dustin Trail
Postdoc
Sebastian Mergelsberg
Undergraduate Student
Egidio Tentori
Undergraduate Student
-
RELATED OBJECTIVES:
Objective 1.1
Formation and evolution of habitable planets.
Objective 4.1
Earth's early biosphere.
Objective 4.3
Effects of extraterrestrial events upon the biosphere