2011 Annual Science Report
 Pennsylvania State University
Reporting | SEP 2010 – AUG 2011
Pennsylvania State University
Reporting | SEP 2010 – AUG 2011
Biosignatures in Ancient Rocks
Project Summary
The Earth’s Archean and Proterozoic eons offer the best opportunity for investigating a microbial world, such as might be found elsewhere in the cosmos. The ancient record on Earth provides an opportunity to see what geochemical signatures are produced by microbial life and how these signatures are preserved over geologic time. As part of our integrated plan, we will study geochemical, isotopic, and sedimentary signatures of life in order to understand the context in which these biosignatures formed.
Project Progress
Ohmoto Group
Members: Hiroshi Ohmoto (Co-PI), Yumiko Watanabe (Research Associate), Jamie Brainard (Ph. D. Candidate), Hiroshi Hamasaki (MS Candidate), Andrew Choney (Senior Undergraduate; Research Assistant), and Thomas Rushton (Senior Undergraduate)
During the period 9/1/10 – 8/31/11, Ohmoto group has further advanced the following three controversial theories concerning the chemical and biological evolution of the (early) Earth: (1) Anomalous isotope fractionations of sulfur (AIF-S) in sedimentary rocks were generated by reactions between solid organic matter and aqueous sulfate under hydrothermal conditions, rather than by photochemical reactions; (2) Geochemical cycles of redox sensitive elements (e.g., Fe, U, Mo, Ce, S, and C) through the continental crust – oceans – oceanic crust – mantle systems have been essentially be the same as today since ~3.5 Ga; and (3) Methane hydrates did not contribute to climatic changes, but played an important role in mass extinctions during geologic history.
I. Anomalous isotope fractionations of sulfur (AIF-S) in sedimentary rocks were generated by reactions between solid organic matter and aqueous sulfate under hydrothermal conditions, rather than by photochemical reactions:
The current paradigm for the AIF-S states that: (1) UV photolysis of volcanic SO2 in an O2-poor atmosphere (i.e., _p_O2 < 10-5 PAL) created the AIF-S signatures in sedimentary rocks; therefore, (2) the presence of AIF-S signatures in pre-2.4 Ga sedimentary rocks and the virtual absence of AIF-S in younger rocks are the best evidence for a dramatic change from an anoxic to oxic atmosphere (i.e., the Great Oxidation Event, G.O.E.) at ~2.4 Ga. A major focus of research by Ohmoto group for the past ~10 years has been directed to evaluate the validity of this paradigm through theoretical, experimental, and field-related investigations.
1.2. AIF-S during thermochemical sulfate reduction (TSR) by amino acids and other organic matter: We (Watanabe et al.) reported in Science, April 2009 that reactions between amino acid crystals and aqueous sulfate at 150-200°C generated polysulfide-like sulfur (i.e., Cr-reducible sulfur species) in the amino acid crystals and H2S with variable AIF-S values. Subsequently, Dr. Watanabe and Mr. Andrew Choney (Research Assistant) have been conducting TSR experiments using a variety of solid organic compounds (e.g., mixtures of alanine and glycine; dried cyanobacteria; extracted kerogen from young sediments). They have recognized that the mixtures of two amino acids (alanine and glycine) generated H2S with Δ33S values as large as +2.3‰ and Δ36S values ranging from -1 to +1. 5‰; these values are larger than those produced by alanine or glycine alone. This suggests the TSR with multiple organic compounds may generate larger Δ33S values. Most (> 90 percent) of the AIF-S values in pre-2.4 Ga sedimentary rocks have Δ33S and Δ36S values less than those obtained in our experiments. Also most sedimentary rocks with AIF-S signatures contain abundant kerogen and pyrite, which were affected by high-temperature hydrothermal fluids. For these reasons, we have proposed that TSR, rather than atmospheric UV reactions, produced most, if not all, of the AIF-S signatures in pre-2.4 Ga sedimentary rocks (Watanabe et al., 2010; Otake and Watanabe, in press; Watanabe et al., in prep.).
The TSR experiments using dried cyanobacteria remnants and kerogen extracts from the Green River shales produced complicated results because they originally contained high amounts of organic-sulfur (~5 wt%). We observed that bitumen and H2S-bearing gas were liberated at temperatures as low as ~100°C without additional SO42-. From the change in the δ34S values of H2S during the reactions between the kerogenh and SO42-, we have recognized that TSR by natural kerogen occur at temperatures as low as ~200°C. The Δ33S values of the H2S remained at < ±0.2‰ during these experiments. However, we expect the Δ33S values to increase with reaction time and temperature.
Our suggestion for the origin of AIF-S in geologic samples (i.e., by TSR utilizing solid organic matter, rather than the UV photolysis of SO2 in an O2-poor atmosphere) is consistent with other recent studies: (1) thermodynamic calculations suggesting that SO2 was not an important volcanic gas before ~2.4 Ga (Kump and Barley, 2008; Ohmoto et al., 2010; Halevy et al., 2010); (2) the discovery of AIF-S signatures in ~1.8 Ga black shales in Finland (Young et al., 2009); and (3) the discovery of AIF-S signatures in modern aerosols generated by burning of pyrite-rich coals (Ding et al., 2006).
1.2. AIF-S during carbon – SO2 reactions: As a part of his MS thesis research, Mr. Hiroshi Hamasaki investigated the details of chemical and isotopic processes during reactions between solid organic matter and oxidized sulfur species (e.g., SO42-, SO2) using activated carbon (AC) and SO[~2](g)] at 200 and 250°C. He has recognized that the chemical reactions proceed through: (1) formation of weakly-bound SO2 molecules on the AC surfaces; (2) formation of strongly-bound SO2-] bearing surface molecules; (3) formation of sulfate-like S-O-C molecules in the AC; (4) reduction of the S-O-C species to polysulfide-like S-C species in the AC; and (5) reduction of polysulfide-like S-C species to sulfide-like S-C species. If H2O was present in the environments, step (6), desorption of the S-C species as H2S, would have occurred. We have observed large kinetic isotope fractionations, ranging from ~+12‰ to ~-8‰ in δ34S and up to +0.14‰ in Δ33S values. These isotopic characteristics are similar to those of H2S (and other S-bearing compounds) in petroleum, natural gas, and some ore-forming solutions, suggesting that the natural TSR probably occurred by solid C-bearing compounds (e.g., dead bodies of (micro)organisms and kerogen), rather than by gaseous, aqueous, or liquid C-bearing compounds.
II. Geochemical cycles of redox sensitive elements (e.g., Fe, U, Mo, Ce, S, and C) through the continental crust – oceans – oceanic crust – mantle systems have been essentially the same as today since ~3.5 Ga:
The current paradigm for the early Earth states that the geochemical cycles of redox sensitive elements during the Archean eon were entirely different from those today: that is, Fe was removed from, but U, Mo, Ce, S and C were retained in, soils during subaerial weathering, and that the oceans were rich in Fe, but poor in U, Mo, Ce and S, because the atmosphere was presumably O2 poor. However, from the mineralogical, chemical and isotopic investigations of submarine basalts, chert-jaspers, barite deposits, volcanogenic massive sulfide deposits, and paleosols of Archean ages, we have recognized that the geochemical cycles of redox-sensitive elements during the Archean were probably the same as today.
2.1. Evidence in 3.46 Ga submarine basalts: The Archean Biosphere Drilling Project (ABDP) recovered a 260 m-long drill core section (ABDP #1) at Marble Bar, Pilbara, Western Australia. It encompasses one of the world’s oldest (3.46 Ga) jasper formation (i.e., low-grade banded iron formation) and associated submarine basalts. We (Hoashi et al., 2009, Nature Geosciences) reported that the hematite crystals in the jaspers formed at T >60°C from the mixtures of Fe2+-rich submarine hydrothermal fluids (T = 150-250°C) and O2-rich deep ocean water. Subsequent research by our group (Ohmoto et al., 2010 & 2011; Ohmoto et al., in review by Nature Geoscience) has led to a discovery that the jaspers and submarine basalts in ABDP #1 core exhibits significant enrichments of ferric-iron (FeIII), U, Mo, and Li, and positive and negative anomalies in cerium (Ce) concentrations, which are essentially the same as the characteristics of modern jasperoids and submarine basalts that were formed by reactions with oxygenated deep ocean water. These data suggest that by 3.46 Ga, the atmosphere and oceans were fully oxygenated, the oceans were Fe-poor, but U-, Mo- and Li-rich, large continental masses had developed, and the modern-styled geochemical cycles of redox-sensitive elements were established. Subduction of FeIII- and U-enriched oceanic crust may have created large-scale heterogeneity of the mantle since ~3.4 Ga, including the “lead (Pb) paradoxes”, where the mantle contains more radiogenic Pb and higher U/Th ratios than the bulk Earth. Therefore, through the creation of the oxygenated oceans and atmosphere, oxygenic photoautotrophic organisms have influenced the geochemistry of the deep Earth and the nature of volcanism since ~3.5 Ga ago.
2.2. Evidence in ~3.2 Ga massive sulfide deposits: The Panorama district of the Pilbara Craton, Western Australia hosts several volcanogenic massive sulfide deposits (VMS) of ~3.2 Ga in age. Our field investigation in the summer of 2010 has confirmed the reports by Australian geologists (e.g., Brauhart et al., 2001; Huston et al., 2001) that these VMS deposits and the associated submarine volcanic rocks possess essentially the same mineralogical and geochemical characteristics as those of Phanerozoic in ages, such as the increases in ferric/ferrous iron ratios and the abundance of barite. This suggests that the deep-ocean water at 3.2 Ga had essentially the same O2 and SO42- contents as the modern ocean water. Mr. Choney investigated the mineralogy and geochemistry of barite-rich core samples from this district as a part of his senior thesis (Choney, 2011). Mr. Jamie Brainard has been investigating the mineralogy, elemental geochemistry, and isotope geochemistry (S, Li, Fe, Cr, etc) of submarine volcanic rocks from this district as a part of his Ph.D. thesis research.
2.3. Evidence in the ~2.76 Ga Mt. Roe paleosol: This paleosol, occurring in the Western Pilbara Craton, Western Australia, was intensely studied by Holland group of Harvard University (e.g., Macfarlane et al., 1994; Rye and Holland, 2000; Yang et al., 2002), and it has been regarded by many geologists as the best example of paleosols that formed under an anoxic atmosphere because of the loss of ΣFe from the paleosol section. ABDP #6 hole was drilled to recover a complete section of the Mt. Roe paleosol, which has not been modified by modern weathering. A preliminary mineralogical and geochemical investigation of the drill core sample by Mr. Tom Rushton as a part of his senior thesis research (Rushton, 2011) has revealed that the behaviors of Fe, Cr, and S in the 2.76 Ga Mt. Roe paleosols are essentially identical to those in paleosols of Phanerozoic ages, suggesting that these soils formed by organic acid-rich soil waters under an O2-rich atmosphere. It implies that the land surface was fully colonized by microbes, including cyanobacteria, aeronic heterotrophs, and anaerobic heterotrophs.
III. Methane hydrates did not contribute to climatic changes, but played an important role in mass extinctions during geologic history:
Researchers have suggested that the release of CH4 from the marine methane hydrate zone (MHZ) has caused the global warming and mass extinctions during geologic history. Yet, no comprehensive quantitative assessment has been made of the amounts of global CH4 release due to changing ocean conditions. We (Brainard and Ohmoto, 2010, 2011; Brainard and Ohmoto, in review by Nature Climate Change) have computed the global methane inventory based on our evaluation of the MHZ distribution in the global oceans as a function of the seafloor depth and bottom water temperature. The results suggest that the maximum carbon© fluxes by CH4 from the global MHZ will be <~0.33 Gt/yr due to the bottom-water temperature rise at a rate of <1°C/1,000yrs, and <~0.03 Gt/yr due to a sea-level drop at <100m/10,000yrs. These values are comparable to or less than the C fluxes of ~0.4Gt/yr by anthropogenic CH4 and ~0.2 Gt/yr by biogenic CH4 (e.g., by methanogenic bacteria and termite). Taking into consideration of the recent findings that the greenhouse effect of CH4 is much less than previously thought and that most of the methane liberated from the MHZ may not escape to the atmosphere, we suggest the role of marine methane hydrates in changing the climate during geologic history was probably negligible.
However, the atmospheric O2 level could have dropped to <80% of the present level to cause the mass extinction of the land-based plants and animals during the Permian-Triassic (P-T) transition and possibly during the other extinction periods through the following chain of events: (1) an increase in the bottom water-temperature of ~6-12°C or a sea level drop of 100 m during a ~20 Ma period; (2) creation of anoxic seas (e.g., Tethys) by scavenging of dissolved O2 by rising methane bubbles from the MHZ; (3) development of euxinic seas with increased activity of sulfate reducing bacteria; (4) a rise of sulfidic water mass and release of H2S to the surface zone to kill the majority of photoautotrophs; (5) a decrease in the primary productivity to <1/7 of the present value to lower the long-term O2 production flux to below the present level; (6) an increased O2 consumption flux by soil due to an increase in surface temperature; (7) a combined effect of (5) and (6) to decrease the atmospheric pO2 to <80% of the present atmospheric level.
Hedges Group
Summary
Hedges continued his research on the refinement of the timescale of life, with emphasis on relating the origin of different groups of organisms to chemical and geological biomarkers. The scope of this work has been extended to the species level in prokaryotes and eukaryotes, and the group is developing new methods for analysis of large sequence data sets. Additional efforts have been devoted towards expansion of the TimeTree web resource (http://www.timetree.org/) and associated smartphone and tablet applications, with major releases already in 2011.
Progress report
The NAI research of Blair Hedges mainly concerns the refinement of the tree of life scaled to geologic time (i.e., the timetree of life). This information helps to relate chemical and geological biomarkers in the rock record to organisms that may have produced those biomarkers. In turn this provides a better understanding of biosphere evolution, and identifying possibly universal mechanisms and pathways applicable to life on other planets. In early 2009 the Hedges lab produced 12 publications bearing on this goal, including an edited book covering all of life down to the family level (S. B. Hedges & S. Kumar, The Timetree of Life, Oxford U Press). Since that project, research in the Hedges Lab has expanded, and now focuses on microbial evolution to the species level, which include thousands of prokaryote and eukaryote species (manuscripts in progress). A NASA Planetary
Biology Intern, Katherine Hargreaves (PhD student from the University of Manchester, UK), was hosted in 2011 and she assisted in this research.
In parallel to this empirical research we have continued development of a web database and resource, TimeTree (http://www.timetree.org/, now holding divergence data from 1200 published studies. In 2011, we released Version 2.0 of TimeTree on the web, and a new version of the popular iPhone/iPod app that was first released in 2010. We also released, in 2011, a new app for the iPad (HD) tablet, and one for Android smartphones. The free apps all contain a detailed geological timescale using standard colors and allow users to visualize all molecular data points in this reference scale, and explore more detailed information for each data point. TimeTree receives >1000 searches per day and is used as a teaching tool in high schools. In 2011, Hedges also directed, for the seventh season, the Astrobiology Summer Program (NSF-REU Site), which funds undergraduates from universities and colleges across the U.S. to come to Penn State and astrobiology research with PSARC members.
Kump Group
Members: Lee Kump, PI; Ying Cui, Ph.D. student; Kyle Rybacki, Ph.D. student; Chris Junium, Ph.D. student in the Arthur group; Stamatina Hunter, M.S. student, Garrison Loope, M.S. student; Genming Luo, Ph.D. student/collaborator from China University of Geosciences, Wuhan) has focused primarily on three fronts this year: causes and aftermath of the end-Permian mass extinction, mechanisms and consequences of the Great Oxidation Event, and Green Lake, NY as a modern analogue of Proterozoic oceans.
Research on the Great Oxidation Event and Paleozoic Anoxia/Euxinia
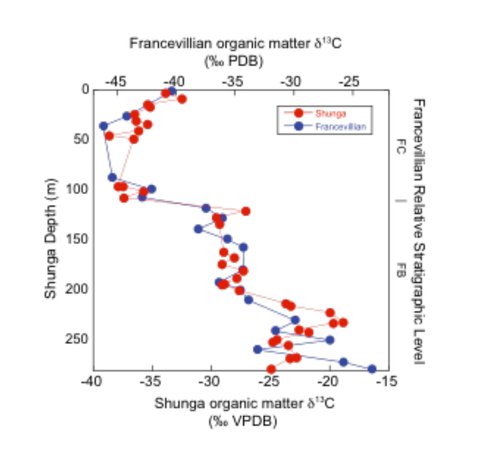
Kump, Arthur, Junium and Luo, funded by NAI and NSF, together with colleagues in the ICDP FAR-DEEP drilling program from Scotland and Norway, have discovered a massive oxidation event at what is traditionally considered the end of the “Great Oxidation Event”, about 2.0 billion years ago. The evidence is a very large, negative carbon isotope excursion preserved in both organic matter and carbonates from Fennoscandia (in FAR-DEEP drill core) and also, strikingly, in Gabon, Africa (Fig. 1) that we have labeled the “Shunga-Francevillian Carbon Isotope Anomaly”. The required input of oxidized organic matter (presumably from sedimentary rock weathering) is huge, perhaps reflecting the initial, deep penetration of highly oxidizing groundwaters in Earth history (Kump et al., Science, in review).
Figure 1. Comparison of bulk organic matter δ13C values from the Onega Basin (“Shunga”) of Fennoscandia (FAR-DEEP cores 12A and 12B) and the Francevillian Basin of Gabon.
New Ph.D. student Kyle Rybacki is beginning to investigate other evidence for deep crustal oxidation during the Shunga-Francevillian carbon isotope anomaly. He is focusing on the anomalous Kuetsjarvi lavas of Fennoscandia, anomalous in that they have a very high Fe(III)/Fe(II) ratio possibly reflecting an initially highly oxidized lava, but clearly influenced by oxidizing fluids during/after eruption, including hematite-filled amygdules (vesicles from the original lava).
In support of the notion that crustal oxidation began in earnest during the GOE, Konhauser et al. (2011; Kump as co-author and including ASU team member Tim Lyons, UC Riverside) have found evidence for the onset of microbial pyrite oxidation and sulfuric acid generation during weathering in the earlier stages of the GOE.
Extending this work into the Paleozoic, Kump has collaborated with others including Tim Lyons (ASU team member, UC Riverside) and Bruce Runnegar (UCLA Team) on isotopic evidence for anoxia and euxinia (buildup of hydrogen sulfide) in Cambrian oceans (Gill et al., 2011; Saltzman et al., 2011).
Research on the end-Permian mass extinction
Progress on the end-Permian extinction event was particularly catalyzed in the last couple years through collaboration with colleagues from the China University of Geosciences, Wuhan, including the visit by Ph.D. student Genming Luo. Genming published two co-authored papers during 2011, one that compiled carbon isotope data from around the world to demonstrate that the first step in the negative anomaly preceded the extinction event (Luo et al., 2011a), and another that argued for an intensification of nitrogen fixation in the aftermath of the extinction event, consistent with other evidence for persistent anoxia in the marine realm (Luo et al., 2011b).
Presently, Ph.D. student Ying Cui is involved in two end-Permian investigations: 1) Field work in South China investigating terrestrial sections for fossil evidence of land ecosystem extinction, for evidence from siderite nodules in paleosols of significant climate changes during the extinction event (as predicted by the Siberian Traps kill mechanism), and for carbon isotope evidence for the negative shift described above, in part to allow for correlation with marine sections; and 2) Modeling of the end-Permian event, using the same approach she used for the Paleocene-Eocene Thermal Maximum. She uses the observed isotope record to force an Earth system model of intermediate complexity, the result being an internally consistent estimate of CO2 injection rates together with their environmental consequences. Her initial work was presented at this fall’s Annual Meeting of the Geological Society of America.
Master’s student Garrison Loope is developing redox proxies from carbonate rocks to expand our ability to infer changes in oxygenation, which in the past had largely been restricted to proxies in shales. He is motivated by recent work by the ASU group on redox proxies in carbonates. His approach though involves using paired analyses of iodine content and rare-earth-element patterns to infer local, and perhaps global changes in redox state of the ocean.
Green Lake as a Modern Analog for the Proterozoic Ocean
Katja Meyer’s dissertation work at Penn State, in collaboration with Macalady, Arthur and Freeman and their students, is now published (Meyer et al., 2011). In this paper she relates the observed distribution of phototroph biomarkers in the lake’s water column to what is preserved in the sediments. She finds that in Green Lake, at least, the diagnostic biomarker for green sulfur bacteria (isorenieratene), key in interpreting euxinic environments in the rock record, is not being produced by the GSBs known to flourish in the lake. Instead, okenone from purple sulfur bacteria dominate the sediments. The cyanobacterium Synechococcus, expected to produce the biomarker 2-α-methylhopane, does not do so in Green Lake despite being the predominant cyanobacterium in the lake. Finally, she presents compelling if not conclusive evidence that benthic PSBs produce okenone; there has been a claim in the literature that only planktonic PSBs produce this biomarker. Her work highlights the need for a much-improved understanding of why and under what conditions organisms produce these biomarkers.
Kasting Group
Greenhouse Warming by Nitrous Oxide and Methane in the Proterozoic Eon
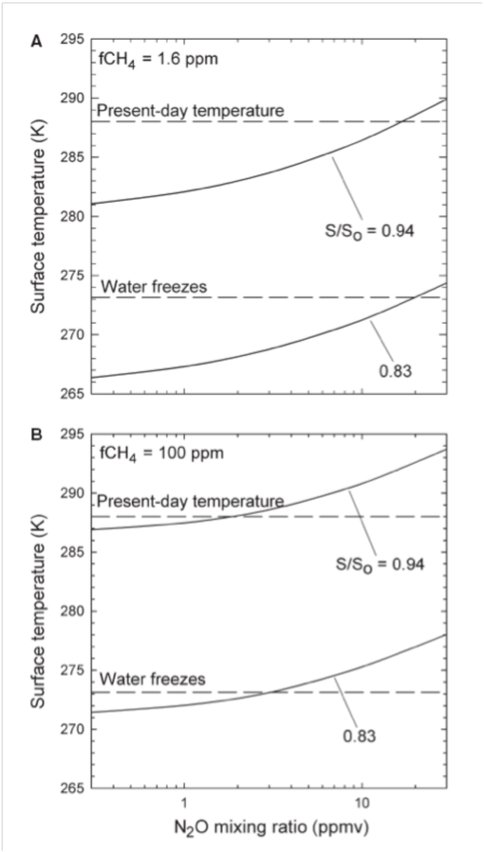
An anoxic, sulfidic ocean that may have existed during the Proterozoic Eon (0.54-2.4 Ga) would have had limited trace metal abundances because of the low solubility of metal sulfides., The lack of copper, in particular, could have had a significant impact on marine denitrification. Copper is needed for the enzyme that controls the final step of denitrification, from N2O to N2. Today, only about 5-6 percent of denitrification results in release of N2O. If all denitrification stopped at N2O during the Proterozoic, the N2O flux could have been 15-20 times higher than today, producing N2O concentrations of several ppmv, but only if O2 levels were relatively high (>0.1 PAL). At lower O2 levels, N2O is rapidly photodissociated. CH4 concentrations may also have been elevated during this time, as has been previously suggested. A lack of dissolved O2 and sulfate in the deep ocean could have produced a high methane flux from marine sediments, as much as 10-20 times today’s methane flux from land. The photochemical lifetime of CH4 increases as more CH4 is added to the atmosphere, so CH4 concentrations of up to 100 ppmv are possible during this time. The combined greenhouse effect of CH4 and N2O could have provided up to 10 degrees of warming, thereby keeping the surface warm during the Proterozoic without necessitating high CO2 levels. A second oxygenation event near the end of the Proterozoic would have resulted in a reductions of both atmospheric N2O and CH4, perhaps triggering the Neoproterozoic “Snowball Earth” glaciations.
Figure 2. a) Global mean surface temperature (K) vs. nitrous oxide abundance. Two curves represent calculations for two solar luminosities: 83% and 94% of present value So. Concentration of CH4 is fixed at 1.6 ppm. (CO2 assumed at preindustrial value of ~320 ppm) b) Global mean surface temperature (K) vs. methane abundance. Two curves represent calculations for two solar luminosities: 83% and 94% of present value So. Concentration of CH4 fixed at 100 ppm.
Availability of O2 and H2O2 on the Pre-photosynthetic Earth
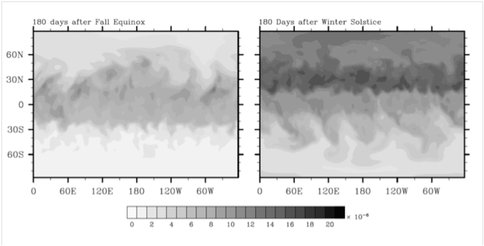
Old arguments that free O2 must have been available at Earth’s surface prior to the origin of photosynthesis have been revived by a new study that shows that aerobic respiration can occur at dissolved oxygen concentrations much lower than had previously been thought, perhaps as low as 0.05 nM, which corresponds to a partial pressure for O2 of about 4×10-8 bar. We used numerical models to study whether such O2 concentrations might have been provided by atmospheric photochemistry. Results show that disproportionation of H2O2 near the surface might have yielded enough O2 to satisfy this constraint. Alternatively, poleward transport of O2 from the equatorial stratosphere into the polar night region, followed by downward transport in the polar vortex, may have brought O2 directly to the surface. Thus, our calculations indicate that this “early respiration” hypothesis might be physically reasonable.
Figure 3. Fraction of tracer ξ/ξ0 at the surface 180 days after Northern autumnal equinox (left panel) and Northern Winter solstice (right panel). Both simulations assume dry conditions and include topography.
Publications
-
Gill, B. C., Lyons, T. W., Young, S. A., Kump, L. R., Knoll, A. H., & Saltzman, M. R. (2011). Geochemical evidence for widespread euxinia in the Later Cambrian ocean. Nature, 469(7328), 80–83. doi:10.1038/nature09700
-
Haqq-Misra, J., Kasting, J. F., & Lee, S. (2011). Availability of O 2 and H 2 O 2 on Pre-Photosynthetic Earth. Astrobiology, 11(4), 293–302. doi:10.1089/ast.2010.0572
-
Hoffmann, M., Hilton-Taylor, C., Angulo, A., Bohm, M., Brooks, T. M., Butchart, S. H. M., … Stuart, S. N. (2010). The Impact of Conservation on the Status of the World’s Vertebrates. Science, 330(6010), 1503–1509. doi:10.1126/science.1194442
-
Konhauser, K. O., Lalonde, S. V., Planavsky, N. J., Pecoits, E., Lyons, T. W., Mojzsis, S. J., … Bekker, A. (2011). Aerobic bacterial pyrite oxidation and acid rock drainage during the Great Oxidation Event. Nature, 478(7369), 369–373. doi:10.1038/nature10511
-
Kumar, S., & Hedges, S. B. (2011). TimeTree2: species divergence times on the iPhone. Bioinformatics, 27(14), 2023–2024. doi:10.1093/bioinformatics/btr315
-
Kump, L. R., Junium, C., Arthur, M. A., Brasier, A., Fallick, A., Melezhik, V., … Luo, G. (2011). Isotopic Evidence for Massive Oxidation of Organic Matter Following the Great Oxidation Event. Science, 334(6063), 1694–1696. doi:10.1126/science.1213999
-
Luo, G., Wang, Y., Algeo, T. J., Kump, L. R., Bai, X., Yang, H., … Xie, S. (2011). Enhanced nitrogen fixation in the immediate aftermath of the latest Permian marine mass extinction. Geology, 39(7), 647–650. doi:10.1130/g32024.1
-
Luo, G., Wang, Y., Yang, H., Algeo, T. J., Kump, L. R., Huang, J., & Xie, S. (2011). Stepwise and large-magnitude negative shift in δ13Ccarb preceded the main marine mass extinction of the Permian–Triassic crisis interval. Palaeogeography, Palaeoclimatology, Palaeoecology, 299(1-2), 70–82. doi:10.1016/j.palaeo.2010.10.035
-
Meyer, K. M., MacAlady, J. L., Fulton, J. M., Kump, L. R., Schaperdoth, I., & Freeman, K. H. (2011). Carotenoid biomarkers as an imperfect reflection of the anoxygenic phototrophic community in meromictic Fayetteville Green Lake. Geobiology, 9(4), 321–329. doi:10.1111/j.1472-4669.2011.00285.x
-
Roberson, A. L., Roadt, J., Halevy, I., & Kasting, J. F. (2011). Greenhouse warming by nitrous oxide and methane in the Proterozoic Eon. Geobiology, 9(4), 313–320. doi:10.1111/j.1472-4669.2011.00286.x
-
Saltzman, M. R., Young, S. A., Kump, L. R., Gill, B. C., Lyons, T. W., & Runnegar, B. (2011). Pulse of atmospheric oxygen during the late Cambrian. Proceedings of the National Academy of Sciences, 108(10), 3876–3881. doi:10.1073/pnas.1011836108
- Brainard, J.L. & Ohmoto, H. (In Review). Effects of climatic and sea-level changes on the marine methane hydrate inventory. Nature Climate Change.
- Hamasaki, H., Watanabe, Y. & Ohmoto, H. (In Preparation). An experimental investigation of multiple sulfur isotope fractionations during heterogeneous reactions between SO2 and activated carbon. Geochimica et Cosmochimica Acta.
- Ohmoto, H., Bevacqua, D.C., Watanabe, Y. & Yamaguchi, K. (In Review). Iron-poor, but uranium- and lithium-rich, Archaean oceans. Nature Geosciences.
- Ohmoto, H., Lasaga, A.C., Watanabe, Y. & Otake, T. (In Preparation). Anomalous isotope fractionations of sulfur. Geochemical.
- Ohmoto, H., Lasaga, A.C., Watanabe, Y. & Yamaguchi, K. (In Preparation). Evidence for the fully-oxygenated oceans and atmosphere during the Archean Eons. Monograph of the Geological Society of America.
- Otake, T. & Watanabe, Y. (In Press). Using multiple sulfur isotopes to understand the Earth’s surface environments during the Archean era. Geochemical.
- Watanabe, Y., Choney, A. & Ohmoto, H. (In Preparation). Multiple isotope fractionations of sulfur during thermochemical sulfate reduction by solid organic matter. Geochimica et Cosmochimica Acta.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Fabia Battistuzzi
Collaborator
James Farquhar
Collaborator
Christopher Junium
Collaborator
Sudhir Kumar
Collaborator
Timothy White
Collaborator
Aubrey Zerkle
Collaborator
James Fulton
Postdoc
Yumiko Watanabe
Research Staff
Ying Cui
Doctoral Student
Hiroshi Hamasaki
Doctoral Student
Matthew Heinicke
Doctoral Student
Lev Horodyskyj
Doctoral Student
Genming Luo
Doctoral Student
Stamatina Hunter
Graduate Student
Ian Johnson
Graduate Student
Nathan Barber
Undergraduate Student
Jamie Brainard
Undergraduate Student
-
RELATED OBJECTIVES:
Objective 1.1
Formation and evolution of habitable planets.
Objective 3.2
Origins and evolution of functional biomolecules
Objective 4.1
Earth's early biosphere.
Objective 4.2
Production of complex life.
Objective 4.3
Effects of extraterrestrial events upon the biosphere
Objective 5.1
Environment-dependent, molecular evolution in microorganisms
Objective 5.2
Co-evolution of microbial communities
Objective 5.3
Biochemical adaptation to extreme environments
Objective 6.1
Effects of environmental changes on microbial ecosystems
Objective 6.2
Adaptation and evolution of life beyond Earth
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems