2011 Annual Science Report
 Montana State University
Reporting | SEP 2010 – AUG 2011
Montana State University
Reporting | SEP 2010 – AUG 2011
Surface Chemistry of Iron-Sulfur Minerals
Project Summary
The exposure of pyrite surfaces to energetic particle beams creates an activated surface that is capable of facilitating the reduction of nitrogen molecules to ammonia. Experimental results and complementary theoretical calculations indicates that the exposure of pyrite surfaces creates anomalously reduced iron atoms. The chemical state of the surface iron atoms is somewhat similar to iron in the active center of several key enzymes. The triple bond in dinitrogen sorbed onto these reduced surface iron atoms weakens, which is a key step in the conversion to ammonia, a key reagent in the formation of amino acids on the prebiotic Earth
Project Progress
The understanding of relevant catalytic chemical transformations on iron-sulfur mineral and particle surfaces requires an understanding of the composition and structure of the surface and its defects. In this project, the surface of pyrite was modified by exposure to a deuterium atom beam and structurally characterized by X-ray absorption spectroscopy (XAS). The reduction of dinitrogen reaction on the treated surface was investigated experimentally using molecular-beam-surface scattering techniques. In addition, in silico molecular models have been developed for iron and sulfur sites of the pyrite surface.
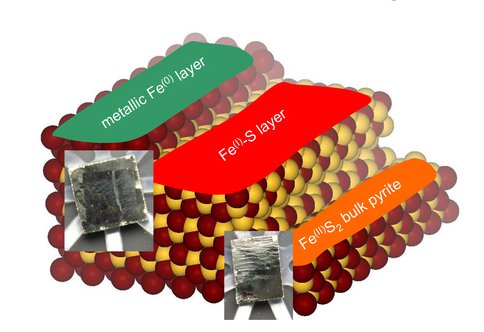
The exposure of a pristine pyrite surface (111 cut) to deuterium atoms leads to the formation of a previously not described, reduced Fe-S phase. The surface composition, oxidation level of iron and sulfur sites, and the arrangement of the surface atoms of the treated pyrite was determined from multi-edge XAS measurement conducted at SLAC National Accelerator Laboratory. The energy position of the X-ray absorption K-edge of iron in the deuterium-treated surface and its Extended X-ray Absorption Fine Structure was compared to several standard materials. The XAS results indicate that the interaction with deuterium leads to a layered system. Bulk pyrite is overlain by a biomimetic Fe(I)-S layer in which the oxidation state of the iron is formally +I. This biomimetic layer is overlain by a metallic iron layer, see figure.
Data obtained using molecular-beam-surface scattering techniques shows that this biomimetic surface facilitates the conversion of dinitrogen to ammonia. These data show that the partially reduced pyrite surface is more active than the resulting top metallic Fe surface. Furthermore, the data show that the activity of the reduced pyrite surface is reversible: oxidation of the surface with atomic oxygen stops the production of ammonia, and re-reduction of the surface with atomic hydrogen restores the production of ammonia.
In order to understand the implications of the role of reduced iron-sulfur surfaces in activation of small molecules and to design future experiments, development of iron and sulfur centered defect site models on a pyrite surface was carried out using molecular graphical and density functional theory methods. These models allows for prediction of the reactivity of various small molecules (CO/CO2, CH4, N2, H2O, H2S) with activated pyrite surface. A simplified single Fe-site model has already been used to evaluate the interaction of nitrate and nitrite with the pyrite surface and be extended to other systems. In parallel, He2+/alpha-particle and H+ ion beam experiments are being conducted to characterize the formation mechanism of the reduced pyrite surface.
Publications
-
Che, L., Gardenghi, D. J., Szilagyi, R. K., & Minton, T. K. (2011). Production of a Biomimetic Fe (I) -S Phase on Pyrite by Atomic Hydrogen Beam Surface Reactive Scattering. Langmuir, 27(11), 6814–6821. doi:10.1021/la2002833
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Alexios Grigoropoulos
Postdoc
Che Li
Postdoc
Michael Vance
Postdoc
David Gardenghi
Doctoral Student
Logan Giles
Doctoral Student
Travis Harris
Doctoral Student
Bradley Towey
Doctoral Student
-
RELATED OBJECTIVES:
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules
Objective 3.3
Origins of energy transduction
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems