2011 Annual Science Report
 Montana State University
Reporting | SEP 2010 – AUG 2011
Montana State University
Reporting | SEP 2010 – AUG 2011
Ecology of Extreme Environments: Characterization of Energy Flow, Bioenergetics, and Biodiversity in Early Earth Analog Ecosystems
Project Summary
The distribution of organisms and their metabolic functions on Earth is rooted, at least in part, to the numerous adaptive radiations that have resulted in the ability to occupy new ecological niches through evolutionary time. Such responses are recorded in extant organismal geographic distribution patterns (e.g., habitat range), as well as in the genetic record of organisms. The extreme variation in the geochemical composition of present day hydrothermal environments is likely to encompass many of those that were present on early Earth, when key metabolic processes are thought to have evolved. Environments such Yellowstone National Park (YNP), Wyoming harbor >12,000 geothermal features that vary widely in temperature and geochemical composition. Such environments provide a field laboratory for examining the tendency for guilds of organisms to inhabit particular ecological niches and to define the range of geochemical conditions tolerated by that functional guild (i.e., habitat range or zone of habitability). In this aim, we are examining the distribution and diversity of genes that encode for target metalloproteins in YNP environments that harbor geochemical properties that are thought to be similar to those that characterize early Earth. Using a number of newly developed computational approaches, we have been able to deduce the primary environmental parameters that constrain the distribution of a number of functional processes and which underpin their diversity. Such information is central to constraining the parameter space of environment types that are likely to have facilitated the emergence of these metal-based biocatalysts.
Project Progress
Our hydrothermal ecology studies have taken one of three paths that either employ i) community ecological approaches to the study the distribution and diversity of target protein encoding genes, ii) shotgun approaches to examine community level metabolic composition, or iii) activity-based studies to evaluate hypotheses derived from i and ii above using either in situ microcosm based studies or pure cultures.
In our community ecology thrust area, we have successfully identified the environmental constraints that have defined the evolutionary trajectory of nitrogenase, the mercuric reductase, and genes involved in the biosynthesis of chlorophyll. In the case of nitrogenase, we determined a pervasive distribution of this gene product across a number of geochemical gradients in YNP. The phylogenetic diversity of this gene product appears to be constrained by a combination of salinity and geographic distance. This evinces a role for both ecology and geography in structuring the biodiversity of nitrogenase in hydrothermal ecosystems. The pervasive distribution nitrogenase was unexpected and in particular, an elevated abundance of nitrogenase genes in environments characterized by extremes of high temperature and low pH was noted. These environments are characterized by elevated concentrations of ammonia, the product of nitrogenase, which would be expected to inhibit the activity of these organisms and constrain there distribution. We therefore examined whether these environments supported active populations of diazotrophic (i.e., N2 fixing) organisms. These environments were found to harbor a significant population of acidiphilic diazotrophs.
In collaboration with members of the ASU Follow the Elements group as well as collaborators from Pennsylvania State University, we examined the distribution of genes involved in chlorophyll biosynthesis, as well as light-harvesting pigments produced by these organisms, along geochemical gradients in YNP. We found a correspondence between the genetic diversity of organisms involved in the synthesis of pigments, and the distribution of pigments. These genes were non-randomly distributed in YNP along gradients characterized by temperature, pH, and sulfide. We evaluated the role of these environmental constraints in defining the habitable zone for this functional process. The results indicate that photosynthesis is constrained to environments with temperatures less than 73°C. Below this temperature range, other geochemical variables including sulfide appear to suppress photosynthesis to lower temperature realms due to their inhibitory effects on photosynthetic activity.
We have also initiated several new collaborations with investigators from the ASU Follow the Elements team and the NASA Ames team aimed at integrating energetic considerations into our community ecological analyses. This includes characterization of environments that support hydrogen-based metabolisms, formate and CO metabolism, as well as light-independent CO2 metabolism. These studies have been primarily conducted with ASU, but were recently expanded to include the many unique geothermal environments in the Great Basin [(Collaboration with scientists from the University of Nevada-Las Vegas (UNLV)]. Preliminary results of in situ microcosm incubations indicated significant CO and formate oxidation activity in a number of intriguing environments in Yellowstone National Park, as well as in alkaline springs in the Great Basin. Likewise, light independent CO2 fixation has been demonstrated in a number of unique hydrothermal environments in Yellowstone, the Great Basin, as well as China.
In addition, we have initiated new studies aimed at examining patterns of biodiversity along vertical gradients in Green Lake (collaboration with scientists at Hamilton College), a meromictic lake characterized by steep redox gradients, as well as the Great Salt Lake, a terminal lake characterized by steep gradients in salinity (collaboration with scientists at Colorado School of Mines and Westminister College). In the latter case, we have also begun characterizing the biodiversity and function of bioherm communities that have formed over long geological periods. These structures are thought to represent analogs of early Earth stromatolites that have been preserved in the geological record. Thus, these studies have the significant potential to inform our understanding of the evolutionary history of the organisms involved in bioherm formation, as well as the biogeochemical functions that they catalyze that result in the formation of these structures.
The extension of ABRC research into sulfur cycling stems from the omnipresence of sulfur species in modern day hydrothermal environments, as well as the perceived importance of sulfur metabolism in early life forms. Using massively parallel computation approaches (collaboration with ASU Follow the Elements), we have determined that the metabolomes (inferred from 20 chemotrophic metagenomes) present in chemotrophic ecosystems in YNP can best be explained by variations in the availability of sulfur in these ecosystems. Furthermore, the energetics of sulfur-based metabolisms is also a significant predictor of community metabolomes from these 20 environments. This suggests that energetic considerations underpin the distribution of metabolism in natural microbial communities. We also examined the role of sulfur metabolism and molecular adaptation in the ecologically relevant sulfur-reducing crenarchaeote Acidilobus sulfurireducens. The results point to a novel role for intermediate sulfur compounds in supporting these chemotrophic communities and reveal novel adaptations to tolerate the extreme acidity and temperature characterizing the environments that this organism inhabits.
Field Work.
Our field expeditions this year have included several day trips to YNP, as well as a two week trip to YNP to coordinate sampling with members of the ASU-Follow the Elements team. This field trip was conducted in order to directly connect field based aqueous, solid, and gas-phase chemical observations conducted by our ASU colleagues with the laboratory-based genetic analyses and the field-based activity assays that we conduct. In addition, an ABRC graduate student accompanied members of the ASU-Follow the Elements team on an expedition to sample geothermal springs in Iceland, as part of an ongoing effort to determine the global biogeographic constraints on organisms that inhabit hydrothermal ecosystems. This year, we also expanded our field work to include a field campaign in the Great Basin of Nevada (collaboration with UNLV scientists). Here, the specific focus was to examine metabolic coupling between several life-sustaining biogeochemical processes. All of the hydrothermal ecology sampling efforts are aimed at furthering our understanding of the habitable zone of processes that were likely important in sustaining early forms of life on Earth.
In addition to hydrothermal ecology field work, ABRC scientists traveled to the Great Salt Lake to sample saturating potassium chloride and sodium chloride brines, in an effort to demonstrate molecular adaptation to salinity. This work was conducted in collaboration with scientists from the United States Geological Survey, Westminister College, and Colorado School of Mines. Particular focus was placed on obtaining representative samples of bioherms and sediments from a gradient of salinity conditions for use in understanding the role of environment in constaining bioherma and sediment biodiversity, function, and/or morphology. Many of these samples were sent to ASU for isotopic characterizations of bulk carbon and nitrogen, which will provide critical insight into differences in the primary metabolic functions that sustain bioherm communities and which might influence morphology.
Publications
Boyd, E.S., K.M. Fecteau, J.R. Havig, E.L. Shock, and J.W. Peters. Modeling the habitat range of phototrophic microorganisms in Yellowstone National Park: Toward the development of a comprehensive fitness landscape. In review. *Corresponding author.
Boyd, E.S. and G.K. Druschel. 2011. The role of intermediate sulfur compounds in the reduction of elemental sulfur by Acidilobus sulfurireducens. In review.
Hamilton,T.L., K. Vogl, D.A. Bryant, E.S. Boyd, and J.W. Peters. 2011. Environmental constraints defining the distribution, composition, and evolution of chlorophototrophs in thermal features of Yellowstone National Pard. Geobiology. 10.1111/j.1472-4669.2011.00296.x.
Wang, Y., E.S. Boyd, S. Crane, P. Lu-Irving, D. Krabbenhoft, S. King, J. Dighton, G. Geesey, and T. Barkay. 2011. The distribution and diversity of the mercuric reductase gene (merA) in microbial communities inhabiting acidic and circumneutral springs in Yellowstone National Park, Wyoming, U.S.A. Microbial Ecology. 10.1007/s00248-011-9890-z.
Hamilton, T.L., R.K. Lange, E.S. Boyd, and J.W. Peters. 2011. Biological nitrogen fixation in acidic high temperature geothermal springs in Yellowstone National Park, Wyoming. Environ. Microbiol. 13: 2204-2215.
Hamilton, T.L., E.S. Boyd, and J.W. Peters. 2011. Environmental constraints underpin the distribution and phylogenetic diversity of nifH in the Yellowstone geothermal complex. Microbiol. Ecol. 61:860–870.
Boyd, E.S., A.D. Anbar, S.R. Miller, T.L. Hamilton, M. Lavin, and J.W. Peters. 2011. A late methanogen origin for the molybdenum-dependent nitrogenase. Geobiology. 9(3):221-232.
Boyd, E.S., A. Pearson, Y. Pi, W.-J. Li, Y.G. Zhang, L. He, C.L. Zhang, and G.G. Geesey. 2011. Physicochemical influences on glycerol dialkyl glycerol tetraether lipid composition in the crenarchaeote Acidilobus sulfurireducens. Extremophiles. 15(1):59–65.
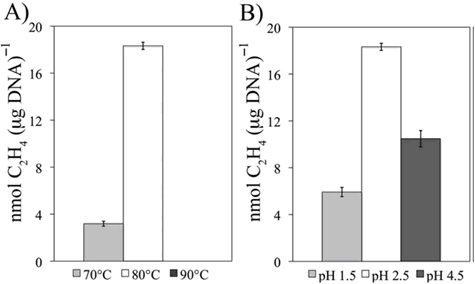
Acetylene reduction activity in nmol C2H4 (mg DNA-1 day-1) associated with sediment biomass sampled from a geothermal spring (~84C, pH 2.5) as a function of altered incubation temperature (panel A) and pH (panel B) of medium. All treatments were run in triplicate, and the standard deviation among replicates is indicated. Acetylene reduction activity was not detected when incubated at 90°C and pH 2.5. The results indicate that the acidiphilic population is specifically adapted to the conditions of the environment from where it was sampled.
Publications
-
Boyd, E. S., Anbar, A. D., Miller, S., Hamilton, T. L., Lavin, M., & Peters, J. W. (2011). A late methanogen origin for molybdenum-dependent nitrogenase. Geobiology, 9(3), 221–232. doi:10.1111/j.1472-4669.2011.00278.x
-
Boyd, E. S., Fecteau, K. M., Havig, J. R., Shock, E. L., & Peters, J. W. (2012). Modeling the Habitat Range of Phototrophs in Yellowstone National Park: Toward the Development of a Comprehensive Fitness Landscape. Frontiers in Microbiology, 3. doi:10.3389/fmicb.2012.00221
-
Hamilton, T. L., Boyd, E. S., & Peters, J. W. (2011). Environmental Constraints Underpin the Distribution and Phylogenetic Diversity of nifH in the Yellowstone Geothermal Complex. Microbial Ecology, 61(4), 860–870. doi:10.1007/s00248-011-9824-9
-
Hamilton, T. L., Lange, R. K., Boyd, E. S., & Peters, J. W. (2011). Biological nitrogen fixation in acidic high-temperature geothermal springs in Yellowstone National Park, Wyoming. Environmental Microbiology, 13(8), 2204–2215. doi:10.1111/j.1462-2920.2011.02475.x
-
Hamilton, T. L., Vogl, K., Bryant, D. A., Boyd, E. S., & Peters, J. W. (2011). Environmental constraints defining the distribution, composition, and evolution of chlorophototrophs in thermal features of Yellowstone National Park. Geobiology, 10(3), 236–249. doi:10.1111/j.1472-4669.2011.00296.x
-
Wang, Y., Boyd, E., Crane, S., Lu-Irving, P., Krabbenhoft, D., King, S., … Barkay, T. (2011). Environmental Conditions Constrain the Distribution and Diversity of Archaeal merA in Yellowstone National Park, Wyoming, U.S.A.. Microbial Ecology, 62(4), 739–752. doi:10.1007/s00248-011-9890-z
- Boyd, E.S. & Druschel, G.K. (In Review). The role of intermediate sulfur compounds in the reduction of elemental sulfur by Acidilobus sulfurireducens.
- Boyd, E.S., Pearson, A., Pi, Y., Li, W-J., Zhang, Y.G., He, L., Zhang, C.L. & Geesey, G.G. (2011). Physicochemical influences on glycerol dialkyl glycerol tetraether lipid composition in the crenarchaeote Acidilobus sulfurireducens. Extremophiles, 15(1): 59–65.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Trinity Hamilton
Doctoral Student
Rachel Lange
Undergraduate Student
Matthew Urschel
Unspecified Role
-
RELATED OBJECTIVES:
Objective 3.2
Origins and evolution of functional biomolecules
Objective 3.3
Origins of energy transduction
Objective 3.4
Origins of cellularity and protobiological systems
Objective 4.1
Earth's early biosphere.
Objective 4.2
Production of complex life.
Objective 5.1
Environment-dependent, molecular evolution in microorganisms
Objective 5.2
Co-evolution of microbial communities
Objective 5.3
Biochemical adaptation to extreme environments

