2011 Annual Science Report
 NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2010 – AUG 2011
NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2010 – AUG 2011
Task 3.4.1 Tholin Chemical Analysis Using Nuclear Magnetic Resonance
Project Summary
The definition and assessment of future flight capable analytical methods for complex organic analysis was pursued, in particular evaluating the potential of nuclear magnetic resonance (NMR).
Project Progress
Co-Investigator Mark Smith and colleagues focused on defining and assessing future flight-capable analytical methods for complex organic analysis, in particular evaluating the potential of nuclear magnetic resonance (NMR). The first step was to prepare Titan tholin-like material for use in evaluating potential analytical flight techniques. This work used the Advanced Light Source and isotopically altered nitrogen/methane mixtures. They observed enhancement of heavy carbon and nitrogen incorporation of the tholins. These effects may be due to optical bleaching phenomena in the dissociative or predissociative states of both molecules and are currently under analysis. However, they have also obtained macroscopic (0.1 – 1 μg) quantities of completely enriched (1H, 13C and 15N) 60 nm and 82.5 nm tholins.
NMR shows tremendous promise for in situ analysis of complex mixtures, possibly avoiding the difficult and uncertain separation protocols suitable for generally unknown mixtures of polar organics. Using the isotopically enriched plasma tholins they investigated 3-dimensional NMR spectra. As a benchmark, they have used the spectra of enriched poly-hydrogen cyanide.
As a test, multinuclear, multidimensional solution-state NMR spectroscopy was employed to investigate structural sub-units in poly-HCN. Using selective isotopic enrichment in 13C and 15N the couplings between all the nuclei in the polymeric mixtures could be identified and from these multidimensional investigations, discrete structural units were identified for the most concentrated small molecular components. The NMR results indicated that the HCN predominately polymerizes to a one-dimensional chain structure (polyimine) in the presence of trace ammonia or triethylamine. They believe that the polymerization of HCN is a base-catalyzed nucleophilic addition reaction and the one-dimensional chains (polyimine) grow by forming new carbon-carbon bonds. This material is shown to be significantly functionally simpler than plasma tholins and is not a suitable analog for Titan haze material.
Few studies have been reported on the characterization of nascent tholin samples by NMR. In 1993, Sagan et al. analyzed the Titan and Jupiter I tholins by solid-state hydrolyzed tholins.4 In the solid-state 13C NMR spectra, broad resonances were observed at 10-60 ppm, near 70 ppm, 115 ppm and 129 ppm. They assigned the signals at 10-60 ppm to saturated carbons (amine or -CH, CH2 and -CH3 groups), the resonances near 70 ppm to hydroxylic carbons (C-OH) or alkyne groups, the peaks around 115 and 129 ppm to nitriles and alkenes, respectively. Pillig et al. described 1H NMR of derivatized tholin produced by soft X-rays.5 The 1H NMR spectrum of the tholin sample is consistent with that of standard adenine. Combining GC-MS and 1H NMR, they provided the example of evidence for nucleobase synthesis in the Titan atmosphere.
Hertkorn et al. have succeeded in analyzing marine dissolved organic matter and unresolved organic fractions in Earth’s atmosphere by multidimensional NMR coupled with very high resolution mass spectrometry.6
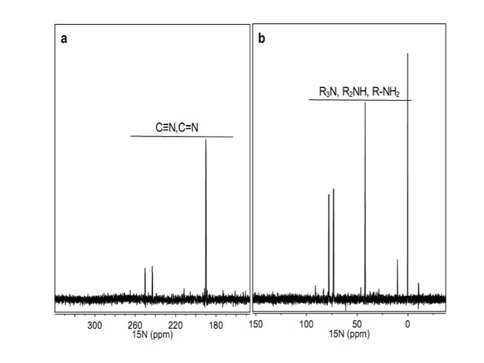
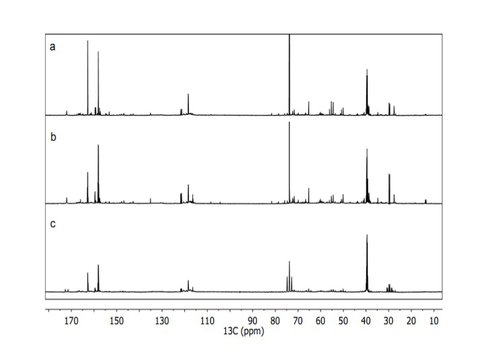
Smith et al. have already reported extensively on their studies of tholin analysis using very high resolution (exact mass) mass spectrometry. This past year they have continued developing their methods to analyze these tholin samples initially using one dimensional NMR techniques. Using 13C and 15N isotope labeled tholins they have carried out the 1-dimensional (1D) NMR experiments on the three nuclei (1H, 13C and 15N) (Figure 1) and developed 1D decoupling NMR experimental methods to help define a functional group inventory of tholins (Figure 2). They are currently exploring the expansion of this to multidimensional NMR methods for the more quantitative determination of refined structures and the extension to photochemical tholins produced by EUV radiation. The chemical shifts of the 1H, 13C and 15N all point to amine, nitrile, imine and N-heteroaromatic functionality being present in the tholin samples. Some structural fragments, such as CH3-C, CH3-N, C-CH2-N, N-CH2-N, CN4, CH2-C≡N, N=CN2, N=C=N, N=CN2 and N=CH-N, are identified using the decoupling experiments.
References:
1 Sagan, C.; Khare, B. N.; Thompson, W. R.; McDonald, G. D.; Wing, M. R.; Bada, J. L., Vo-Dinh, T.; Arakawa, E. T. Astrophys. J. 1993, 414, 399-405.
2 Clarke, D. W.; Joseph, J. C.; Ferris, J. P. Icarus 2000, 147, 282-291.
3 Ramirez, S. I.; Navarro-Gonzalez, R.; Coll, P.; Raulin, F. In Life in the Universe; Seckbach, J., Ed.; Kluwer Academic Publishers; 2004, 281-285.
4 Ruiz-Bermejo, M.; Menor-Salvan, C.; Mateo-Marti, E.; Osuna-Esteban, S.; Martin-Gago, J. A.; Veintemillas-Verdaguer, S. Icarus 2008, 198, 232-241.
5 Pillig, S.; Andragte, D. P. P.; Neto, A. C.; Rittner, R; de Brito, A. N. J. Phys. Chem. A 2009, 113, 11161-11166.
6 Hertkorn, N.; Benner, R.; Frommberger, M.; Schmitt-Kopplin, P.; Witt, M.; Kaiser, K.; Kettrup, A.; Hedges, J. I. Geochim. Cosmochim. Acta 2006, 70, 2990-3010.
Figure 1. Solution-state 15N NMR spectra of Titan tholin in DMSO-d6 with 1H decoupling (chemical shift reference of 15N: δN (NH3) =0 ppm): a) 340 to 140 ppm, b) 160 to -40 ppm.
Figure 2. Solution-state 13C NMR spectra of Titan tholin with different decoupling methods: a) with 1H and 15N decoupling, b) with only 1H decoupling and c) without 1H or 15N decoupling.
-
PROJECT INVESTIGATORS:
-
RELATED OBJECTIVES:
Objective 1.1
Formation and evolution of habitable planets.
Objective 2.2
Outer Solar System exploration
Objective 3.1
Sources of prebiotic materials and catalysts