2011 Annual Science Report
 NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2010 – AUG 2011
NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2010 – AUG 2011
Task 3.1.2 Chemistry Active in Titan Dunes
Project Summary
Triboelectric reactions of complex organics and water ice are a potential chemical mechanism active in the dunes of Titan. Laboratory experiments have been conducted to simulate and assess how important this possibility can be in the Titan context.
Project Progress
Co-Investigator Jack Beauchamp and Graduate Student Daniel Thomas have been investigating triboelectric reactions of complex organics and water ice as a potential chemical mechanism active in the dunes of Titan.
Titan’s N2-CH4 atmosphere provides the starting material for a wide array of organic compounds to be formed via photochemistry, and the presence of unsaturated hydrocarbon, amine, and polycyclic aromatic species has been supported by data from the Cassini-Huygens mission (1-2). Production of tholins by UV irradiation of a simulated N2-CH4 environment has yielded products that match the observed optical properties of Titan haze, suggesting that these compounds provide suitable analogs to Titan aerosol compounds (3-5).
Organics produced in Titan’s atmosphere eventually settle to the surface and very likely contribute to the particulate matter comprising the expansive longitudinal dune features observed at mid-latitudes (6). While the exact composition of the dunes of Titan is unknown, it is likely that they mainly comprise organic and possibly water ice particles approximately 0.2 mm in diameter, the ideal size for saltation by the winds of Titan. Within these dunes, conditions that lead to incorporation of oxygen via contact with water ice or liquid water in Titan’s low temperature environment are of particular interest and have important implications for astrobiology (7-8), particularly since oxygen is present only in trace amounts in Titan’s atmosphere.
One possible source of oxygen incorporation into organics is electric discharge events on Titan’s surface that could possibly lead to dissociation of water molecules or incorporation of oxygen through free radical chemistry. In fact, recent work has demonstrated that electrical discharges occurring over water-ice in a simulated Titan environment do in fact lead to oxygen incorporation via the formation of nitromethane, esters, ethers, and aldehydes (9). These experiments were meant to mimic the possible results of lightning occurring on Titan. While the search for lightning on Titan has to this point been unsuccessful (10), similar electrical discharges may be generated through triboelectrical charging during saltation over the extensive dunes of Titan. In other words, the mechanical energy from wind-driven grains in the dunes of Titan can ultimately drive chemical processes and lead to the incorporation of oxygen into organic compounds via tribochemical reactions (11). In addition, these events would be able to access a significantly larger inventory of organics than that accessed by a rare lightning strike, making the investigation of such processes even more intriguing.
During the saltation process, organic particles undergo charging due to friction between particles (12), and these processes have been measured to create electric fields up to 160 kV/m on Earth (13), well above the fields required to generate electrical discharges even at the higher pressures of Titan’s surface. While the implications of such discharge events for the dust storms of Mars have been extensively studied, the results of such chemistry occurring on the surface of Titan are still wholly unknown. It is likely that free radicals and other reactive species would be generated in localized electrical discharges at particle interfaces (14). These reactive intermediates could initiate processes such as free radical and ionic polymerization that would further transform organics. Of particular interest is the incorporation of oxygen into organic molecules, providing a pathway to the synthesis of biologically relevant compounds. Experiments modeling such systems are being conducted with laboratory-produced tholins and model unsaturated hydrocarbons, nitriles, imines, and aromatic compounds.
An updated fluidized bed system has been constructed to simulate the agitation that particles may experience during saltation on Titan’s surface. As shown schematically in Figure 1a, a flow of nitrogen gas cooled by passing through a coil submerged in liquid N2 is employed to agitate a bed borosilicate glass particles 1 mm in diameter that have been coated with a thin film of an organic compound of interest. The device is shown in operation in Figure 1b. Once the system is cooled, a small amount of liquid water is added to the system to provide a water-ice source for oxygen incorporation. After a short period of agitation, the beads become highly charged, and exhibit increased adhesion to each other and to the glass apparatus (Figure 1c). The improved setup achieves much greater agitation by eliminating the flow-limiting frit at the base of the agitator and by increasing the height available for saltation from 2.5” to 8”. This setup allows for highly charged particles to be removed from the walls during agitation.
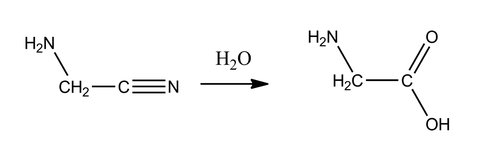
The main molecule studied in the agitation experiments thus far has been aminoacetonitrile, a possible precursor of biologically relevant compounds such as glycine (Reaction 1) that has been detected in laboratory Titan tholin analogues. After several hours of agitation, the beads were rinsed with methanol, and the extracted molecules were analyzed by GC-MS, LC-APCI-MS, and ESI-MS. At this stage, these methods were unable to identify a detectable difference between the control and experimental samples. However, it was determined that the limit of detection of the instruments and methods employed required approximately 1 μmol of product to be present for definitive detection.
Discharge events in such an environment are likely rare, and more sensitive detection as well as optimization of the fluidized bed operation may be necessary to fully characterize the likelihood that such processes contribute to the inventory of organics with astrobiological significance on Titan’s surface. One possible method being investigated to increase the number of discharge events is to decrease the pressure in the agitation apparatus and thus obtain a higher frequency of discharge and thus more product molecules. A new apparatus to conduct such experiments is currently under investigation in the lab. In addition, the use of polystyrene instead of borosilicate particles is currently being examined, as polystyrene more closely mimics the organic molecules likely to be found in the dunes of Titan and may also provide a greater frequency of discharge events due to its significantly different dielectric properties. In addition the polystyrene particles avoid introduction of oxygen into the products from the support rather than adsorbed water. Finally they are employing two different size particles, seeking to derive advantage from reports that differential charging of the particles can contribute to enhanced electrical discharge activity.
References:
1. A. J. Coates et al., Planetary and Space Science 57, 1866 (2009).
2. F. J. Crary et al., Planetary and Space Science 57, 1847 (2009).
3. B. N. Khare et al., Icarus 60, 127 (1984).
4. S. I. Ramirez et al., Icarus 156, 515 (2002).
5. H. Imanaka, M. A. Smith, Proc. Natl. Acad. Sci. U.S.A. 107, 12423 (July 13, 2010, 2010).
6. R. D. Lorenz et al., Science 312, 724 (May 5, 2006, 2006).
7. D. P. O’Brien, R. D. Lorenz, J. I. Lunine, Icarus 173, 243 (2005).
8. C. D. Neish, A. Somogyi, M. A. Smith, Astrobiology 10, 337 (Apr, 2010).
9. K. Plankensteiner et al., Icarus 187, 616 (2007).
10. G. Fischer, D. A. Gurnett, Geophys. Res. Lett. 38, L08206 (2011).
11. M. K. Beyer, H. Clausen-Schaumann, Chemical Reviews 105, 2921 (2005).
12. J. F. Kok, N. O. Renno, Physical Review Letters 100, 014501 (2008).
13. D. S. Schmidt, R. A. Schmidt, J. D. Dent, J. Geophys. Res. 103, 8997 (1998).
14. C. Kajdas, K. Hiratsuka, Proc. Inst. Mech. Eng., Part J 223, 827 (2009).
 Figure 1. Fluidized bed reactor; (a) Schematic representation of agitation of beads in fluidized bed; (b) current generation fluidized bed reactor in use; (c) evidence for charging of beads as they stick to walls of reactor through electrostatic interactions.
Figure 1. Fluidized bed reactor; (a) Schematic representation of agitation of beads in fluidized bed; (b) current generation fluidized bed reactor in use; (c) evidence for charging of beads as they stick to walls of reactor through electrostatic interactions.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Daniel Thomas
Graduate Student
-
RELATED OBJECTIVES:
Objective 1.1
Formation and evolution of habitable planets.
Objective 2.2
Outer Solar System exploration
Objective 3.1
Sources of prebiotic materials and catalysts
