2011 Annual Science Report
 NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2010 – AUG 2011
NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2010 – AUG 2011
Task 3.1.1 Reactions of Organics With Ices and Mineral Grains
Project Summary
A goal is to determine the potential role of mineral surfaces (i.e. meteorite fragments) in catalyzing reactions on Titan’s surface. There is also the possibility of low-energy electron and visible/UV photon stimulated chemistry on aggregates and organic aerosol surfaces.
Project Progress
Co-Investigator Thomas Orlando and Postdoctoral Fellow Heather Abott-Lyon in collaboration with Co-Investigator Mark Smith sought to determine the potential role of mineral surfaces (i.e., meteorite fragments) in catalyzing reactions on Titan’s surface. They also examined the possibility of low-energy electron and visible/UV photon stimulated chemistry on aggregates and organic aerosol surfaces. They utilized state-of-the art surface science and laser techniques to examine non-thermal reactions on low-temperature ice surfaces and mineral interfaces. The mineral samples were created by laser ablation, and the reactions were examined using surface FTIR-spectroscopy, time-of-flight mass spectroscopy and temperature-programmed desorption/reactions.
Thus far they have examined the interaction of small organic molecules (HCN, C2H2, CH3NH2 and CH3CN) on mineral surfaces (silicates) and on organic and/or water ice substrates. Initial results indicate that reactive proton scattering occurs readily on organic/water ices at cryogenic temperatures during low-energy electron (5-50 eV) bombardment. This leads to the facile formation and ejection of complex polyatomic ions. Silicate minerals and nitrogen-rich tholins also likely play a significant role in the production of pre-biotic molecules since they can act as catalysts and reactive “substrates”. In the case of tholins, these complicated molecules can be the source of ionic species such as C2H3+, C2H5+, CH-, CN- and C2H- as well as reactive radicals such as CH, C2H, CN, etc.
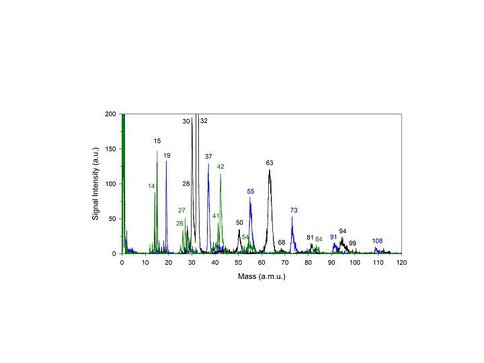
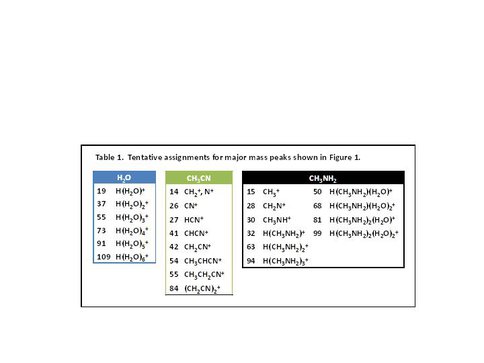
Lab measurements were performed on model organic/water ices (i.e., mixtures of H2O, C2H6, CH3NH2 and CH3CN) under ultrahigh vacuum conditions. Low-energy electrons (< 150 eV) were used to simulate the secondary electrons produced by cosmic rays and were shown to induce proton transfer plus desorption in a single event at the cryogenic temperature of 55 K, see Figure 1. In Table 1 the identity of the mass peaks with the most probable candidates is shown.
Threshold energies for cluster production and desorption via electron-stimulated desorption (ESD) from methylamine ice (not shown) suggest that electrons directly ionize water adsorbed from the background gas. Based on experimental conditions, the coverage of accumulated H2O is much less than a monolayer. Subsequently, proton transfer from the ionized H2O to a neighboring CH3NH2 results in the detected cation signal. This effect is most apparent on methylamine, which has a higher proton affinity than water in the gas phase. In contrast, acetontrile appears to ionize and form cations without interacting with H2O from the background gas.
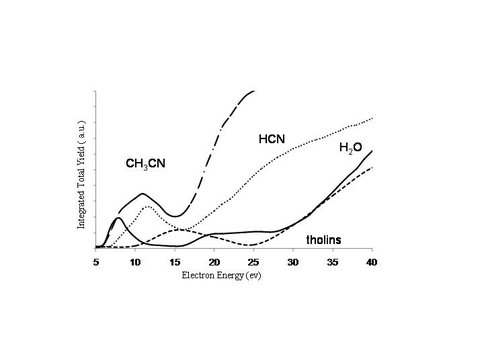
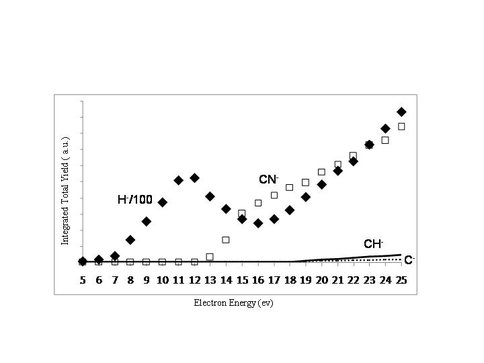
Ices of increasingly higher C/N ratios have been used as aerosol surrogates to provide fundamental information regarding prebiotic chemistry and negative ion formation. Time-of-flight spectra of negative ions from multilayer HCN, CH3CN and tholins on graphite and SiO2 at 120 K have recently been obtained by ESD. In Figure 2, the major anion observed is H- from dissociative electron attachment (DEA). If the electron energy is sufficiently large (>10 eV) dipolar dissociation can occur and produce large molecular anionic fragments, as seen in Figure 3. These DEA resonances will leave behind radicals in the ices that can catalyze polymerization reactions. These data can provide insight into the mechanism and activation energies for the formation of possible prebiotic molecules in the Titan environment.
Figure 1. ESD-TOF spectra for single component ices of H2O (blue, ~60 L dose), CH3CN (green, ~60 L dose) and CH3NH2 (black, ~2 L dose) at 55 K. Spectra were obtained using 150 eV electrons and a total electron flux of ~3×1010 electrons/mm2.
Figure 2. H- DEA resonances from H2O, HCN, CH3CN and tholins.
Figure 3. Molecular anions desorbing during ESD of HCN on SiO2 at 120 K.
Table 1. Tentative assignments for major mass peaks shown in Figure 1.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Mark Smith
Collaborator
Heather Abbott
Postdoc
-
RELATED OBJECTIVES:
Objective 1.1
Formation and evolution of habitable planets.
Objective 2.2
Outer Solar System exploration
Objective 3.1
Sources of prebiotic materials and catalysts