2011 Annual Science Report
 NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2010 – AUG 2011
NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2010 – AUG 2011
Task 2.2.2 Ultraviolet/infrared Spectroscopy and Photoprocessing of Ice Films
Project Summary
Only near-ultraviolet light at long wavelengths can penetrate to the deep Titan atmosphere, not being absorbed by atmospheric gas-phase species. Large hydrocarbons can absorb at these longer wavelengths. Condensed onto atmospheric particles, such hydrocarbons can undergo photochemical reactions initiated by absorption of near-ultraviolet photons.
Project Progress
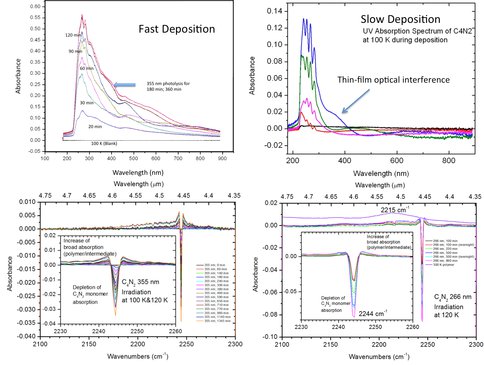
Co-Investigator Murthy Gudipati working with Postdoctoral Fellows Ronen Jacovi and Antti Lignell and Collaborator Isabelle Couturier successfully carried out the synthesis of dicyanoacetylene (C4N2)—a potential component of Titan’s aerosol—and purified it for spectroscopic and photochemical studies in their newly established TOAST (Titan Organic Aerosol Synthesis and Spectroscopy) laboratory at JPL. They then pursued detailed photochemical studies on C4N2 as ice film (simulating condensed aerosol conditions) of ~500 nm thick at 100 K. Analysis was performed with simultaneous UV and IR spectroscopic studies on the same aerosol ice sample. In order to derive quantitative data, they had to optimize deposition conditions that led to optically high-quality UV spectra. For example, when deposited quickly over 15 minutes at 10-5 mbar pressure of C4N2, the UV spectra obtained were not of sufficient quality. However, deposition of C4N2 at 10-7 mbar for over 2 hours did lead to excellent UV absorption spectra of the C4N2 aerosol ice films, as shown in Figure 1. They obtained UV and IR spectra of C4N2 simultaneously that match well with the literature values. Subsequently, they conducted photochemistry experiments with various visible and UV lasers that are defocused to avoid multiphoton processes at wavelengths: 532 nm, 355 nm, and 266 nm. Photon fluxes were typically ~50 mW/cm2. Irradiation at 532 nm did not result in any changes in ice UV and IR absorption spectra, whereas with 355 nm they could clearly see depletion of C4N2 monomer bands at ~3% over 18 hours of irradiation. In contrast, photochemical depletion of C4N2 ice film with 266 nm laser was much faster, depleting over 80% of C4N2 in 3 hours of photolysis. They also conducted the same studies at the University of Provence, Marseille, where under very similar conditions the solid aerosol ice films were irradiated with a 150 W high-pressure Mercury lamp equipped with a glass filter that removed UV light at wavelengths shortward of 320 nm. During an overnight irradiation, about 50% of C4N2 was depleted.
When the ice films were warmed up, beyond 160 K C4N2 sublimes off, leaving a nonvolatile residue that is persistent at room temperature (300 K). This residue, shown in Figure 2, clearly resembles Titan’s tholins and does not disappear under vacuum over several days. These studies unambiguously show that longer wavelength photochemistry of condensed Titan organics can occur at lower haze altitudes (~100 km) in Titan’s atmosphere and that the non-volatile organic polymeric material resembles closely the conventional “tholins”, both in its physical appearance (color) as well as infrared spectral features.
Figure 1: Top: UV absorption spectra of C4N2 aerosol ice films deposited slowly (right) and fast (left). Faster deposition results in highly scattering aerosols. Bottom: Longer wavelength photodepletion (chemistry) of C4N2 ice films at 355 nm (left) and 266 nm (right) laser wavelengths. The non-volatile organic residue (tholin-like material) absorbs at 2215 cm-1, indicating that a part of the CN group remains in the polymeric structure.
Figure 2: Longer wavelength (355 nm followed by 266 nm) laser photo-polymerization product “Titan Tholin” under vacuum (top three pictures), and exposed to atmosphere (bottom left), in methanol solution (bottom right), and the KBr window after cleaning the “UV-photo-tholin” (bottom middle). Color of these polymeric non-volatile organic residue is very similar to Titan’s haze and Titan tholins prepared through other procedures such as discharge.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Isabelle Couturier
Collaborator
Ronen Jacovi
Postdoc
Antti Lignell
Postdoc
-
RELATED OBJECTIVES:
Objective 1.1
Formation and evolution of habitable planets.
Objective 2.2
Outer Solar System exploration
Objective 3.1
Sources of prebiotic materials and catalysts